Q. Use of Halides of Boron?
Boron trihalides of the type BX3 exist for all the four halogens. Boron trifluoride can be prepared on a large scale by the fluorination of boric oxide or borax with fluoaspar and conc. H2S04:
B203 + 3CaF2 + 3H2S04-------------------> - 2BF3 + 3CaS04, + 3H20
Na2B4O7 + 6CaF2 + 8H2S04 ---------------> 4BF3 + 6CaS04, + 6NaHS04, + 7H20
In the laboratory, pure BF3 is best prepared by thermal decomposition of Gene diazonium tetrafluoroborate, PhN2BF4:
PhN2BF4-----------------------------------------> PhF + N2 + BF3
BCI3, and BBr3, are prepared on a large-scale by direct halogenation of B2O3 in the presence of C, e:g:
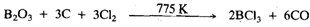
In the laboratory BCl3 and BBr3, are prepared by halogen exchange reaction between BF3 and Al2X6:
2BF3+ Al2X6----------------------> 2BX3+ 2AlF3 where X = Cl and Br
BI3 is prepared in good yield by reacting-LiBH4, or NaBH4, with I2 at 400 and 475 K, respectively:
LiBH4, + 4l2-------------------------------> Bl3 + LiI + 4HI