Thin layer chromatography (TLC)
Detection of excessive levels of one or more amino acids in blood plasma or in urine is often crucial for diagnosis of a group of genetic (inherited) disorders that cause abnormal amino acid metabolism. These are rare conditions that can be very serious if untreated, but they do not generally show obvious clinical symptoms at birth therefore early diagnosis is vital to allow prompt treatment.
Diagnosis of amino acid metabolic disorders examines any excessive levels of amino acids in the plasma of babies using a small blood sample taken a few days after birth. A common and reliable method employs an analytical separation technique known as thin layer chromatography (TLC) that allows amino acids to be separated from each other on the basis of their different side chains, which influence a range of parameters such as overall size, charge, solubility and affinity (to the TLC plate).
Experimental
The TLC plate consists of a thin layer of solid silica on a foil support, onto which small samples of blood plasma are loaded, each at a carefully measured starting point. Amino acids in the plasma are separated by means of a solvent that moves up the silica layer through capillary action, and carries the amino acids with it. Different amino acids move at different rates, dictated by the chemical composition of their side chains. After the solvent has moved up the plate, amino acids are visualised using the stain ninhidryn. Normal blood plasma will not contain any amino acids at excess levels, and reveals no staining.
You will be provided with simulated newborn blood plasma samples. One sample will be from a healthy infant (Sample N) and three will be from infants with known amino acid disorders; citrullinemia (Sample C), maple syrup urine disease (Sample M) and phenylketonuria (Sample P). You will use these samples as controls against which to examine a sample from a new patient (Sample X), in order to try and diagnose any condition this patient may have.
You need to wear gloves at all times when handling the TLC plate
Use the schematic on the next page as a guide
1. Load 5 samples onto a carefully measured baseline on the TLC plate
- Measuring accurately, a make small pencil mark on each edge, 1cm from the narrow end of the plate
- DO NOT draw a pencil line across the plate - it will score the silica and your samples won't run
- Dip a micro-capillary pipette into one of your plasma samples and look carefully to see that liquid has been drawn up into the pipette through capillary action. The liquids are colourless, so look very closely for the tiny meniscus line.
- Holding the micro-capillary pipette vertically, touch it gently onto the TLC silica coating, directly in line with your pencil marks.
- Do not press hard - capillary action should draw the liquid out onto the silica, leaving you with a small wet spot.
- Use a fresh capillary tube for your remaining samples, and try to load equal amounts of each. Make sure you record the order of samples across the plate in your lab book.
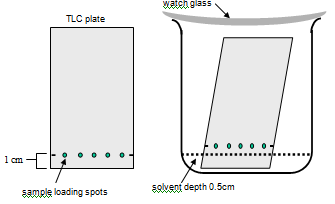
Stand the TLC plate in its solvent solution and allow it to run undisturbed
- Pour a small volume of eluting solvent [35% butan-1-ol, 10% acetic acid] into your beaker to reach a depth of 0.5cm.
- Gently lower the TLC plate into it - the plate should stand in the solvent reservoir, but your sample loading line should be above the level of the liquid.
- THINK - don't pour the solvent in with the plate already in the beaker - you'll splash it and ruin the experiment.
- Put a watch glass on top of the jar (otherwise we will all pass out eventually as a result of breathing the solvent!) and allow the solvent to move up the silica by capillary action.
3. Stain the TLC plate with ninhydrin and incubate to develop the colour
- When the solvent front reaches 1cm from the top of the TLC plate (usually 50-60min), remove it (wearing gloves!) from the jar.
- Re-cover the jar with the watch glass. Sit the TLC plate on top to dry briefly (5-10 min).
- Mark the position of the solvent front using a pencil and take your plate to a demonstrator for spraying with the ninhydrin stain(wearing gloves!).
- Place the sprayed plate in the hot oven for ~10min to dry and allow the stain to develop.
Recording
You will not be able to permanently add the TLC plate to your lab notebook. Apart from the fact there is only one per team, the solvent and stain both contain chemicals that would eventually degrade the pages of your book. Instead, you should make an accurately-measured figure to represent your plate and the information on it. Think carefully about how you will display, annotate and describe this diagram - look at a range of figures within your textbooks to guide you. Your assessors will be looking for accuracy in terms of scale, measurements, labels and descriptions, and for clarity in the figure itself and the information (results) it portrays.
Interpretation
Patients with an amino acid metabolic disorder will accumulate one or more amino acids to high levels in their blood, and this will show as one or more coloured spots on the stained TLC plate. Differences in equipment, chemical suppliers and individuals performing any one experiment lead to variations in the results. Because every TLC plate will run differently, the absolute distance of each spot from its point of origin will vary slightly every time. However, the behaviour of each amino acid in a particular solvent silica is consistent, therefore the relative distance moved from its point of origin compared to the distance moved by the solvent front from that same point will also be constant.
This is known as the Rf or Retention factor and is calculated very simply, as follows
Rf = [distance moved by spot from loading point (mm)] /distance moved by solvent from loading point (mm)]
The Rf value ensures accurate diagnosis irrespective of which lab or scientist performs the test.