Thermodynamic process - Thermodynamics:
Thermodynamic system undergoes changes because of the energy and mass interactions. Thermodynamic state of system changes because of these interactions.
The mode in which change of state of system takes place is termed as PROCESS such as constant pressure, constant volume process etc. In the figure given below process 1-2 & 3-4 is constant pressure process whereas 2-3 & 4-1 is constant volume process.
Take gas contained in cylinder and being heated up. The heating of gas in cylinder shall result in change in state of gas as its pressure, temperature etc. shall increase. But, mode in which this change of state in gas takes place during heating shall constant volume mode and thus the process shall be called as constant volume heating process.
The PATH refers to series of state changes through which system passes during a process. Therefore, path refers to the loci of various intermediate states passed through by system during process.
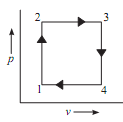
CYCLE refers to characteristic sequence of processes in such a fashion that initial and final states are identical to each other. Thus, a cycle is one in which the processes occur one after other so as to finally, land
the system at same state. Thermodynamic path in cycle is in closed loop form. After the occurrence of cyclic process, system shall show no sign of processes having occurred. It can be said that cyclic integral of any property in a cycle is zero.
1-2 & 3-4 = Constant volume Process
2-3 &4-1 = Constant pressure Process
1-2, 2-3, 3-4 & 4-1 = Path
1-2-3-4-1 = Cycle