The calorific value of a fuel can be theoretically determined by using dulong's formula. It is assumed that heat evolved comes from the combustion of carbon, hydrogen, and sulphur present in the fuel. The total heat evolved is equal of heat evolved by the combustion of individual constituents present.
The calorific value of hydrogen = 34500 cal/gm.
Calorific value of carbon = 8080 cal/gm
Calorific value of sulphur = 2240 cal/gm.
Then, dulong's formula is HCV =
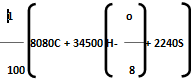
Where, C, H, O, S are percentage fractional weight of carbon, hydrogen, oxygen and sulphur respectively obtained from the analysis of 1 gm of the fuel.
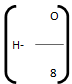 represents the available hydrogen.
represents the available hydrogen.
It is assumed that oxygen present in fuel combines with hydrogen to form water. 8 parts by weight of oxygen combines with one part by weight of hydrogen, to form water. So actual heating value of hydrogen is obtained by above expression.