State the law of radioactive decay. Show that radioactive decay is exponential in nature. Does the activity of a radioactive element depend on external physical conditions?
Law of radioactive Decay: This law states that the number of radioactive nuclei decreases exponentially with time. The rate of disintegration dN/dt, i.e., the number of atoms disintegrated per second is directly proportional to the number of atoms present (N) a that moment. This is known as Decay Law.
Explanation
Let N0 be the number of atoms present in a radioactive sample initially i.e., at t = 0.
Let N be the no of atoms left at a time t and dN be the no of atoms disintegrating in a short interval of time t.
The rate of disintegration = dN/dt
According to decay law = dN/dt α N or dN/dt = -λN
Where, λ = decay or disintegration constant. The negative sign indicates that as time
increases, the number of atoms decreases
dN/dt= -λN OR dN/N = -λdt
Integrating the above equation
∫dN/N = -λ∫dt or logeN = -λt +c ...(1)
Where c = integration constant and it can be found in the initial condition.
i.e., If t = 0, N = N0
Then, logeN0 = c ............ (2)
From equation (1)
LogeN = - λt + logeN0
LogeN - logeN0 = -λt (or)
Loge(N/N0)= e- λt
Or N= N0e- λt
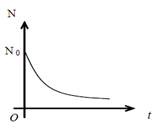
a) The number of radioactive nuclei decreases exponentially with time and reduced to zero after infinite time
b) The above equation represent radioactive decay is exponential in nature. The activity of a radioactivity element does not depend on external physical conditions like temperature, pressure etc.