In the cases that we carried out in lectures we assumed that, in scaling up:
If the TIP Speed was constant then 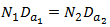 (Note: Because Da and Dt are constructed at a fixed ratio we can also write,
(Note: Because Da and Dt are constructed at a fixed ratio we can also write,

We also showed that when considering the Power/Unit Volume
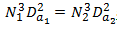 and because Da and Dt are constructed at a fixed ratio we can also write,
and because Da and Dt are constructed at a fixed ratio we can also write, 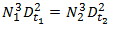
This gives 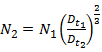
Use the same agitation system as in CASE 1 and the previously given values of Dt, Da, W and J. This tank now needs to be scaled up to a vessel that has a volume Z times as large.This has to be done for the following requirements:
(a) Equal rates of mass transfer for the production of the fine chemical are required. This is often required for certain types of organic synthesis. Mass Transfer is usually occurring between solid-liquid phases or liquid-liquid phases
(b) Equal liquid motion is needed. This is often required for kinetically controlled systems where liquid blending is occurring.
When you have produced the scale up dimensions using geometric similarity, calculate the Power/Unit Volume for cases (a) and (b) and discuss the answers.
Studies show that for equal mass transfer in scale up that the 2/3 power equation should be used and for equal liquid motion the 1.0 power equation should be used. In doing the scale up it is appropriate to use the Length Ratio (Length Factor) as used in the second Case Study in Lectures.