Range of Results: In an experiment to determine the freezing point of octadecanoic (stearic) acid by cooling, some of the acid was melted in a small test-tube and a 100°C thermometer was placed in the test-tube. The thermometer was packed in the test-tube with cotton wool and the test-tube was mounted in a flask, thus preventing cooling. The experimental set up is illustrated in Figure.
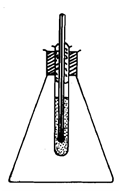
Figure: An experimental set up for determining the freezing point of octadeconic acid.
The temperature was noted every 30 seconds and a graph plotted of temperature against time. The graph is illustrated in Figure.
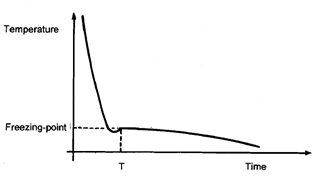
Figure: A temperature/time graph with a 30 seconds gap between readings.
Looking at the graph, you can see that if the temperature was recorded every 30 seconds only up to time T, it would be impossible to establish where the freezing point of octadecanoic acid was. Similarly, if the temperature was only recorded after time T, you could not be certain exactly where the freezing point was. Thus time must be recorded for a wide range of values in order to be able to determine the freezing point accurately. However, appropriate time intervals must also be used. Consider what would happen if you recorded- the temperature every 5 minutes instead of every 30 seconds.
You would probably end up with a graph like the one shown in Figure. Not only would you have trouble judging where the freezing point was from this graph, you would entirely miss the unexpected shape of the curve in Figure just before time T.
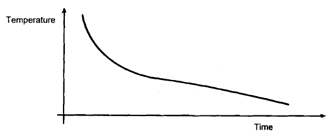
Figure: A temperature/time graph using a 5-minute gap between successive readings.
Conversely, it would be impracticable to record the temperature every 10 seconds, and it would certainly be pointless beyond time T. However, it might be beneficial to obtain more results before time T by more frequent measurement in order to obtain a more accurate plot of the cooling and super cooling curve.
You will see that a similar argument applies to the range of the temperature results. There would be little point heating the acid to, say 250°C, because this will not help you in your study of the freezing point of octadecanoic acid (70°C); and in any case the 100°C thermometer would not be of much use at this high temperature. Similarly there is also no point in making elaborate arrangements to cool the acid to -30°C.
It is important, then, that you ensure that the range of results recorded in an experiment ensures that results' are:
1. Accurate: within the practical limits of experimentation and instrumentation.
2. Useful: covering the areas fundamental to the final analysis of the experiment.
3. Complete: not so inadequate in one part of an experiment as to prevent you making more than a partial analysis of results.
Consideration of' these points is essential at the planning stage of an experiment. The range of results dictates, to a large extent, the apparatus you would use in an experiment. In our example, for instance, you would not use a thermocouple for measuring the temperature, and you would ensure that whatever timepiece was used indicated seconds and minutes.