PROCEDURE : Visit the preparation room of the lab (Physics/Chemistry/Zoology/I3otany), you studied in Exercise 1 and note its features. To make your study easy and to guide you, we have listed below the main features along with some hints so that you can pay attention to them in a systematic way and do not miss out essential aspects. You can also consult, if you feel the need, the lab technician associated with the lab for information and help. You are expected to observe the various aspects and features and note them in the columns we have provided. You are also expected to add your own comments, additional information and suggestions whenever you feel the need, keeping in mind what you have learnt. In some cases the columns may prove inadequate for your Manual notes. In such cases use additional sheets and makes appropriate columns.
1. Location and layout : Note the location of the preparation room with respect to the laboratory. Is the location satisfactory for efficient functioning? Is it located between two labs? How many labs does it service? Draw a floor plan of the preparation room and the labs it provides service to. Draw the position of the wet and dry bench, water tap etc. whatever you observe in the room somewhat similar to the figure shown here
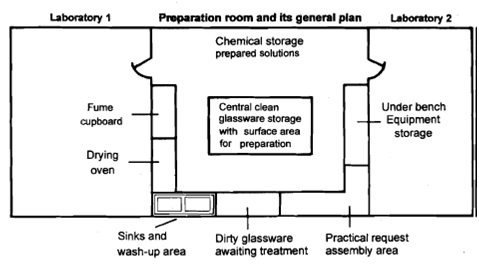
2. Entry and exit points: Note the entry and exit points of the preparation room and specify whether the access and entry is restricted and how. Also comment on the convenience and safety of the entry and exit points.
3. Infrastructure and Organisation: Give a broad list of the types of things that are kept in the preparation room, their number and use. Also describe how they are kept and how often they are used. All the things listed here may not be in the preparation room that you have taken for your study. For example a physics lab may not have chemicals and a chemistry lab may not have stains. These headings are for your convenience. You can change these headings if need be or leave them blank.
4. Storage: Note the kind of storage and racks present in the preparation room. Is there enough room for storage? Pay attention to under bench storage also. Note the position of shelves (in the centre of room, along the walls etc.).
5. Provision for services-gas, water and electricity and drainage: Observe the kinds of the services that are available in the preparation room and how they are provided (through walls/floor/ movable service station). Note the number of outlets of these services and whether they appear adequate. Observe also the plumbing network and drainage as well as condition of taps (if leaky).
6. Windows and ventilation: Note the number and size of windows and provision for forced ventilation in the form of exhaust fans or air-conditioners. Also note if the preparation room is secure from thieves and vandals.
7. Lighting, Heating and Cooling : Observe and note (i) if the room is receiving sufficient natural light (ii). Also observe if there are adequate arrangements for PASL (Permanent Artificial Supplementary Light)? Keeping in view the changing weather condition (in certain parts of the country), also note the kind of arrangements that are provided in the preparation room to maintain comfortable temperature.