Polymerization
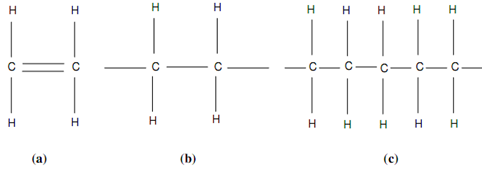
Whether similar molecules join in long chain is termed as polymerization and resulting outcome is a polymer in a chemical reaction. Each plastic are polymers. This was stated that a molecule of ethylene can polymerise while several of these molecules connect in a chain structure as demonstrated in figure of Polyethylene Polymer. Ethylene or C2H4 is an unsaturated molecule since it has a twice bond among two atoms of carbon. This dual bond is again demonstrated in following figure (a). In the presence of a catalyst, pressure or heat the carbon-carbon dual bond is obtained and opens up for bonding along with another activated molecule of ethylene as following figure (b). This procedure of joining may continue until a few hundred to a few thousand molecules have connected in chain. The number of units or mers that join within polymerisation is termed as degree of polymerisation or DP. The polymerisation chain of ethylene is demonstrated in following figure (c) that is same as shown in previous figure of Polyethylene Polymer. This polymer is called as polyethylene and has average DP of 3500 to 25000. The polymer's molecular mass is the product of degree of polymerisation and molecular mass of one unit of the polymer. For illustration, the molecular mass of C2H4 is 2 × 12 + 4 × 1= 28 units. The molecular mass therefore, of polyethylene of DP of 5000 is 28 × 5000 = 140000 units.
Following figure demonstrates an ethylene monomer along with four hydrogen atoms replaced by radicals R1, R2, R3 and R4. Different polymers are acquired by placing such radicals as H, Cl, CH3, COOH3, C6H5, and so on in place of R1, R2, R3 and R4.
While R1 = R2 = R3 = R4 = H, polyethylene is obtained, polyethylene has been demonstrated in figure of Polyethylene Polymer and figure of A Monomer. It is generally utilized for household, tubes and sheets containers.
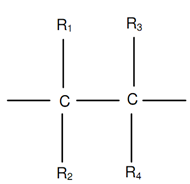
Figure: A Monomer
While R1 = R2 = R3 and R4 = Cl as shown in figure of Polyvinyl Chloride or PVC, the vinyl polymer, termed as polyvinyl chloride or PVC, is acquired. Polyvinyl Chloride is largely employed for conduit pipes and electrical insulation.
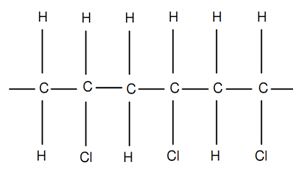
Figure: Polyvinyl Chloride or PVC
If R1 = R2 = H, R3 = CH3 and R4 = COOH3 a polymer termed as polymethyl-methacrylate is acquired. This polymer, often referred to as PMMA, is demonstrated in following figure.
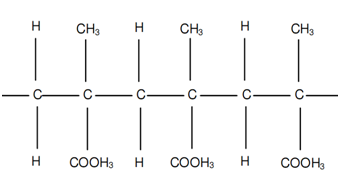
Figure: Poly-methyl-meth-acrylate or PMMA
Poly-methyl-meth-acrylate is commercially called as perspecs, lucite or plexiglass. This is strong and hard but is generally used for its glass as like transparency.
Styrofoam, largely termed as for its porous structure and thermal insulation is acquired when R1 = R2 = H and R3 = R4 = C6H5. It is employed as insulator in buildings and refrigerators and as sound absorbent.
PTFE or polytetrafluoroethylene, commercially called as Teflon, is chemically resistant to each chemical except hydrofluoric acid. This polymer is acquired by replacing each R via fluorine radical, F. Teflon can retain its hardness upto relatively high temperature and thus employed as coating on razor blades, frying pans and for biomedical applications. This is also employed as bearing and vacuum ring.