Orthoboric acid H3BO3 commonly known as boricacid and Meta boric acid HB03, are two well-known and important oxoacids of boron. On a large scale, H3BO3 is prepared by the action of HCl or H2S04 on a concentrated solution of borax:
Na2B07 + 2HCl + 5H20--------------------------> 4H3B03 + 2NaCl
Boric acid is a flaky, white crystalline solid. It is moderately soluble in water. Boric acid is a very weak monobasic acid (pK = 9.25), because it acts as an electron pair acceptor (Lewis acid) from OH rather than as a proton donor (Arrhenius acid).

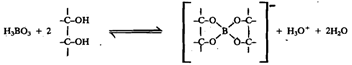
Its acid strength is considerably enhanced on complex formation with polyhydric alcohols such as glycerol and mannitol. With mannitol K drops to 5.15 indicating an b: increase in acid strength by a factor of more than 1.0.
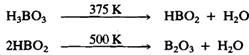
On heating boric acid at 375 K, Meta boric acid, HB02 is formed. On further heating above 500 K, B2O3 is formed:
In solution metaboric acid changes into orthoboric acid.
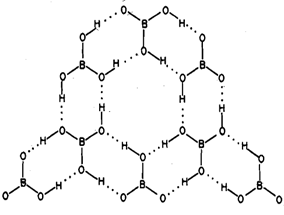
Boric acid has a two dimensional layer structure in which planar B03 units are linked to each other by unsymmetrical hydrogen bonds as shown in Fig. 6.7. In contrast to the short 0-H....O distance of 272 pm within the plane, the distance between consecutive layers is 318 pm. This is the cause of slippery and waxy feel of boric acid which is also a good lubricant.