Nickel cadmium batteries -Construction:
The plates of a nickel cadmium battery are made by sintering a nickel plated steel screen with nickel carbonyl powder. The resultant plaques are then impregnated with the active materials, Nickel salts on the positive, cadmium salts on the negative. The plaques are then placed in electrolyte and subjected to a small current to convert them to their final form.
After washing and drying the plaques are cut into plates, each one having a nickel tab welded to it. The plates are then stacked alternately to produce a cell.
Whilst producing the stack a continuous separator is wound between the plates to prevent them shorting.
Terminals are then welded to the plates and the stack up is inserted into its container, which is sealed and pressure tested.
The separator used is normally a triple layer type, one layer of cellophane, two of woven nylon cloth. Cellophane is used because it has a low resistance and is a good barrier material, it prevents metal particles from shorting the plates whilst allowing current to flow. The cellophane also acts as a gas barrier, preventing oxygen given off by the positive plate during overcharge, from passing to the negative plates. At the negative plates the oxygen combines with the cadmium, reducing the cell voltage and producing heat.
The electrolyte, a solution of potassium hydroxide and distilled water, with a SG of between 1240 and 1300, is then injected into the cell under a vacuum. Fitted to the top of each cell is a special vent that allows the escape of gas but prevents electrolyte spillage.
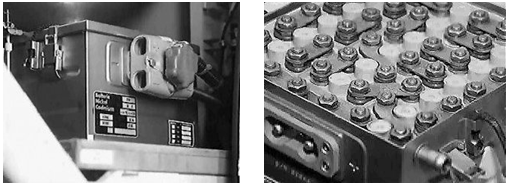
In a typical Ni Cad battery the cells are mounted in a metal case that incorporates 2 venting outlets, carrying handles, a quick release connector and a lid. Each cell is separated from its neighbour by its moulded plastic case and electrically connected by nickel plated steel links between the terminals.