Lead acid batteries:
Lead acid cells have a nominal voltage of 2 Volts, therefore a typical 24V aircraft battery would consist of 12 cells connected in series. The active material in the positive plates is Lead Peroxide (Pb02) the negative plates, Spongy Lead (Pb). The electrolyte is dilute sulphuric acid (2H2SO4).
Construction:
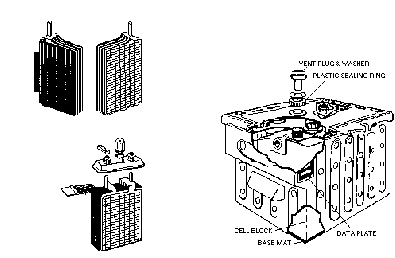
There are two forms of Lead Acid battery construction, conventional and solid block, often referred to as a Varley type battery.
In the conventional battery the plates consist of lead grids into which the active materials are pressed. The positive and negative plates are then interleaved and connected to a lug that forms both a mechanical support and the terminal.
Cells are generally constructed with an additional negative plate, making both outside plates negative. This ensures that chemical action takes place on both sides of each positive plate. When chemical action only takes place on one side of a positive plate it tends to buckle.
The plate arrangement is then inserted into a composite material container which is fitted with a lid. The inside of the container is ribbed to provide additional support for the plates, which are raised clear of the bottom of the container to prevent shorting by any sediment that forms.
To provide further support for the plates and to ensure they cannot touch, separators are fitted, these were originally cedar wood but modern batteries use micro-porous plastic materials.
Each cell is fitted with a special non spill valve that allows gasses to escape, but prevents the spillage of electrolyte, this valve can be removed for checking and adjusting the electrolyte level.
The electrolyte used is sulphuric acid diluted with pure distilled water, the specific gravity of the electrolyte used is determined by the manufacturer, however, it is generally lower than 1300.