Mixture of gasoline and air is used as fuel in internal combustion engine. It is highly compressed and then ignited.
1. The process involves four strokes :
2. The fuel air mixture is drawn into cylinder-(suction stroke).
3. The fuel air mixture is compressed-(compression stroke).
4. The fuel air mixture is ignited by an electric spark. The gases produced due to combustion increase the pressure and push the piston down-(power stroke).
5. The piston moves up and expels the exhaust gases from the cylinder-(exhaust stroke).
The flame on ignition should spread rapidly and smoothly through the gaseous mixture for maximum efficiency of the engine. But sometimes, due to high compression, the gasoline air mixture gets heated to a temperature, so that there is spontaneous combustion before regular sparking. This is called premature ignition. There may be self ignition of last portion of the fuel-air mixture after sparking, resulting in an explosive violence. The pre- mature ignition and decayed ignition causes knocking (i.e. a sharp metallic sound or an explosive violence.)
Knocking causes loss of energy and decreases the efficiency. After ignition, the expansion of gas drives the piston down the cylinder (called suction stoke). When the combustion is complete the piston moves up (compression stroke).

Greater the compression ratio more is the efficiency. The knocking tendency depends on the structure of constituents present.
The order of knocking tendency is straight chain paraffins > branched chain praffins > olefins > cycloparaffins > aromatics.
Knocking also depends on nature of fuel, design of the engines and running conditions etc.
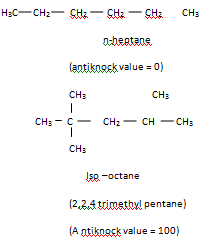
The most commonly used measure of a gasoline's efficiency, to burn without knocking is its octane number. It is found that n-heptane knocks very badly. Whereas iso-octane (2,2, 4-trimethyl pentane) is resistant to knocking. So the octance number of iso-octane is taken as 100, and that of n- heptane is zero.
The octane numbber of a fuel under standard conditions is the percentages of volume of iso-octane in a mixture of iso-octance and n-heptane having the same knocking characteristics as the fuel. For example, gasolone that contain 75% of iso-octance and 25% of n-heptane are given an octance number of 7.5 certain anti-knock quality is reqiired for knock free operation of a gasoline engine. In indias motor, gasoline have number 83.
Antiknocking agents
The knocking tendency can be minimized by adding some compounds known as anti knocking agents. Teraethyllead (Pb(C2H5)4) and iron carbonyl (Fe(CO)5) are used as antiknocking gents. The antiknocking agents retard the rapid combustion of gasoline vapour in the cyclinder. Anyway these are poisonous compounds. Teraethyllead is converted into lead oxide and reacts with any hydrogen peroxide molecules formed, slowing down the chain oxidation reactions. The deposits of lead oxide are harmful to engine life. A small amount of ethylene dibromide (20%) removes lead oxide as volatile lead bromide along with the exhaust gases.
Pb + CH2Br - CH2Br  PbBr2 + CH2 = CH2
PbBr2 + CH2 = CH2
Ethylene dibromide lead bromide ethene
The presence of sulphur compounds in petrol reduces the effectiveness of tetraethayl lead (T.E.L.) in l litre of petrol, about 0.5 ml and in aviation fuel about l ml of E L is added. Aviation gasoline has octane number of 100 or more. It is obtained by comparing it with a mixture of iso- octane and a known quantity of TEL as reference fuel.
Octane number
Fuels required for diesel engine are in contrast to that required for petrol engine.
Knocking in diesel engine
A separate scale is used to grade the diesel oil. The diesel fuel should easily get ignited below compression temperature. The interval between the fuel injection and ignition is termed as induction lag or ignition delay. The induction lag should be shorter for efficient functioning of diesel engine. If the induction lag is shorter, then fuel will burn nonuniformly. i.e. a large portion of fuel gets accumulated in the cylinder and on ignition it burns violently. In contrast to gasoline, diesel engine fuel consists of more straight chain hydrocarbons than violently. In contrast to gasoline, diesel engine fuel consists of more straight chain hydrocarbons than branched and aromatic hydrocarbons. So diesel engine fuels require low ignition temperatures to minimize the induction lag.
Increasing delay period or induction lag occurs in the following order :
n-paraffins < olefins < naphthalenes < isoparaffins < aromatics.
(i.e. reverse as that for antiknocking for gasoline).
n- hexadecane (octane) is given a octane number of 100 as it has a very short ignition delay as compared to any dieselfuel 2- methyl naphthalene is given a octane number zero and has a longer ignition delay compared to any diesel fuel.
The octane number of a diesel fuel may be defined as the percentage of octane in a mixture of octane and 2-methyl naphthalene which will have the same ignition characteristics as the fuel under test, under same conditions.
If a given fuel matches with 40: 60 n-hexadecane and 2-methyl naphthalene, then it is given a octane number of 40.
The octane rating of fuel depends on the nature and composition of hydrocarbons in it. The straight chain hydrocarbons get ignited readily while aromatics do not.
The octane number can be increased on adding additives like ethyl nitrate, acetone etc. other antiknock agents used are benzol, alcohol, tertiary butyl acethyl telluride, tricresyl phosphate etc.
(Note: octane number 100 + 2, means it has the same characteristics as a mixture of iso octance and 2 ml of TEL.)