Inventor claims in adiabatic system:
One inventor claims that 2 kg of air supplied to magic tube at 4 bar and 20°C and the two equal mass streams at 1 bar are produced, one at -20°C and other at 800C. The other inventor claims that it is also possible to produce equal mass streams, one at -40°C and the other at 40°C. Whose claim is correct and why? Consider that it is adiabatic system. (CP air 1.012 kJ/kg K)
Sol: Given that:
Air supplied to magic tube = 2 kg
Inlet condition at magic tube = 4 bar, 20°C
Exit condition: Two equal mass streams, one at - 20°C and other at 80°C for inventor 1. One at - 40°C and other at 40°C for the inventor-II.
Assume that ambient condition 0°C i.e. = 0°C
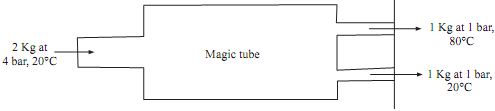
Figure
This process is irreversible; the claim will be correct if the net entropy of the universe (system and surroundings) increases after the process.
Inventor I :
Total entropy at the inlet condition is
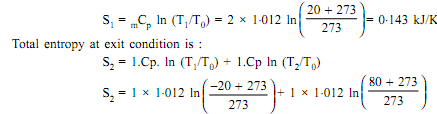
S2 = 0.183
S2 = S1
S2 - S1 > 0
Hnne the claim of inventor is accepatable
For Inventor 2
S2 = 1. CP. ln (T1/T0) + 1. Cp ln (T2/T0)
= 1 x 1.012ln[(- 40 + 273/273)] + 1 x 1.012ln[( 40 + 273)/273]
= - 0.0219KJ/K As S2
S2 - S1 < 0
This violates the second law of thermodynamics. Therefore the claim of inventor is false. - ANS