Q. Illustrate the structure of Alkanes?
Ans.
Alkanes have the general formula CnH2n+2. This means that for every n atoms of carbon, there are 2n + 2 atoms of hydrogen. The first alkane has one carbon and four hydrogens and is called methane:
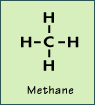
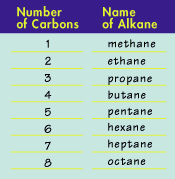
Straight chain alkanes are named according to the number of carbon atoms they have. The first eight alkanes are named in the following table:
Alkyl groups are derivatives from alkanes formed by removal of a hydrogen atom. For example, CH3- takes its name from methane and is called the methyl group.
The symbol R- is used in organic chemistry to represent the "rest of the molecule." It is often an alkyl group. We will see the use of this R- symbol later in this section.
Alkanes are saturated hydrocarbons because they lack double or triple bonds.
Even though alkanes are relatively unreactive, they do undergo some reactions. For example, alkanes burn in air and are important as fuels:
CH4 + 2O2 -> CO2 + 2H2O + Energy
Large alkanes undergo thermal decomposition to give smaller alkanes, alkenes, and hydrogen. This process is called cracking.