Hydrogen Peroxide
Hydrogen peroxide is the other important hydride of oxygen. The oldest method of its preparation is by the action of dilute sulphuric acid on barium peroxide:
BaO2 + H2S04 ------------> BaSO4, + H2O2
H202 when pure, is an almost colourless liquid, freezing at 272.1 K. k is less volatile 0.p. 423 K) than water and somewhat more dense and viscous. It is miscible with water in all proportions. The concentration of aqueous solutions of H202 is expressed in terms of volumes of o48en that will be liberated by a unit volume of H2O2 sample. The two largely used concentrations are 6 volumes and 30 volumes. Let us calculate the percentage of H2O2in a 6 volume sample.
2H2O2--------------->2H2O + O2
0.068kg----------------> 22.4dm3(at STP)
Since the strength of the sample is 6 volumes, 1 dm3 of H202 will produce 6 dm3 of 0, at STP. According to the above relationship 6 dm3 of O2 would be produced by
0.068 x6/22.4 = 0.0182kg H2O2
Thus, I dm3 of a 6 volume sample of H202 will have 0.0182 kg of H2Q2. Hence, its concentration will be 0.0182 x 100 = 1.82 kg per 106 dm3 of sample = 1.82% w/v.
Pure liquid H202 is highly unstable. It decomposes easily; even a speck of dust can initiate explosive decomposition of concentrated solutions.
H202 can act both as an oxidising and a reducing agent. For example, it oxidises ferrous sulphate and lead sulphide to ferric sulphate and lead sulphate, respectively:
2FeSo4 + H2So4 + H2O2-------->Fe2 (SO4)3+ 2H2O
Pbs + 4H2O2-----------> PbSO4+ 4H2O
It reduces chlorine to HCI as shown below:
Cl2+ H2o ------------------> HOCL +HCl
H2O2+ HOCL--------> HCl + H2O + O2
In industry, H2O2, is used mainly for bleaching cotton, wood pulp and other fibers. In the household it finds application as a mild antiseptic and as a bleaching agent.
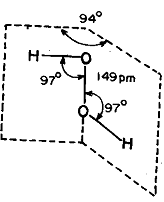
The hydrogen peroxide molecule contains an 0-0 bond and has got a skewed configuration as shown fig.