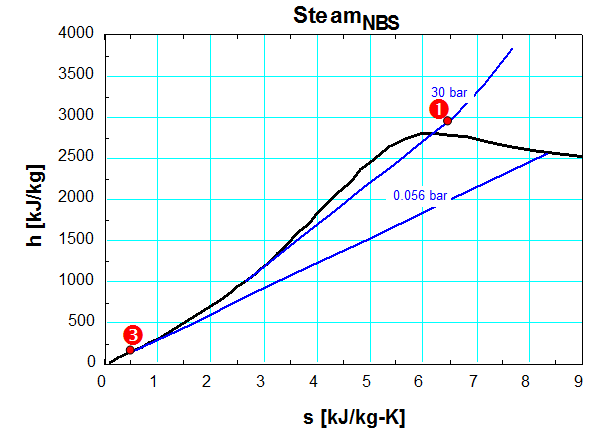
The power plants at Geysers use a dry steam cycle. The steam enters the turbine at 250oC and 30 bars represented by the point 1 in the steam h-s diagram here below. At the outlet the pressure is 0.056 bar with a mix of steam and water. The temperature is 35oC
a. Write the energy balance on the steam turbine. Explain the reasons of the simplifications that you use.
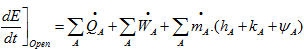
Reasons of simplifications:
- Steady state
- No heat transfer
- No variation of Kinetic Energy or Potential Energy between the inlet and outlet of the steam turbine.
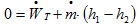
b. Read on the steam h-s diagram the enthalpy at the inlet h1and at the outlet h2_is of the perfect turbine (isentropic).
h1=2,855 kJ/kg
h2_is=1,928 kJ/kg
c. Calculate the power provided by the perfect turbine per kg/s of steam.
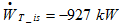
d. At the exit of the turbine, the steam is condensed down to full liquid at the point 3 on the steam h-s diagram before being re-injected in the ground. Read the enthalpy h3 at the outlet of the condenser.
h3=147 kJ/kg
e. The energy variation of the steam between the points 1 and 3 represents the total available energy. What is the conversion rate of the perfect power plant i.e. the ratio of the extracted electric power to the total available energy? Does this number look credible to you?
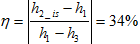
This number is high compared to actual conversion at Geysers but it is based on a perfect turbine.
f. 0.056 bar of steam correspond to a condensation temperature of 35oC. What will happen if the air temperature is higher than 35oC?
If the air temperature is higher than 35oC, the condensation will not happen. The cycle will not work.
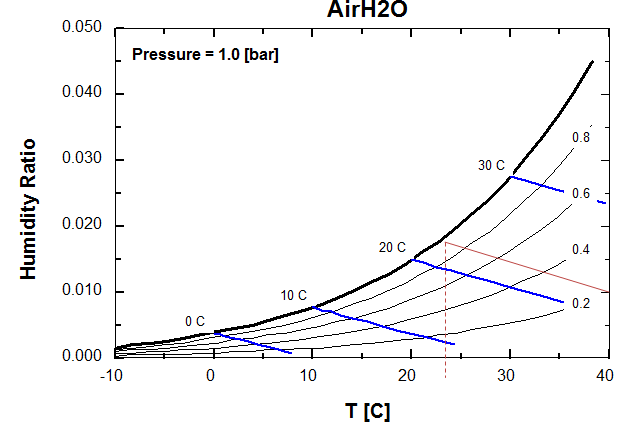
g. How can we improve the performance of the condenser for higher air temperature? Explain using the moisture-air diagram here below.
Using a cooling tower, we will use the evaporation of water to reduce air temperature at the condensor. For example on the graph below if the outside air is at 40oC with a relative humidity of 20%. The water evaporation will lead to saturate the air at 24oC (along an isenthalpic line on the air).