Q. Find out the temperature of Ellingham diagrams?
Ellingham diagrams are very useful for finding out the temperature at which appreciable reaction occurs. The lower the ΔG0 /r graph of an element is on the diagram, the more tables
Its oxide is relative to dissociation into element and oxygen. Such elements will reduce the oxides of other elements that are ΔG0 /r graph appears above them on the diagram at a given temperature. As we have seen above, carbon-reduces ZnO above 1173 K, but below 1173 K zinc will reduce CO. Since the ΔG0 / T graph of C/CO system slopes downwards, it will eventually be below all other graphs at sufficiently high temperatures. Therefore, theoretically carbon will reduce all oxides. But difficulties in obtaining very high temperatures cheaply and the formation of carbides prevent the preparation of the more electropositive metals by this method: It is also clear from the Ellingham diagrams that hydrogen can be used as a reducing agent for the oxides of those elements whose ΔG0 /r graphs are above that of hydrogen in the diagram. Thus hydrogen can reduce the oxides of tungsten, lead, antimony, copper, nickel, zinc and cadmium.
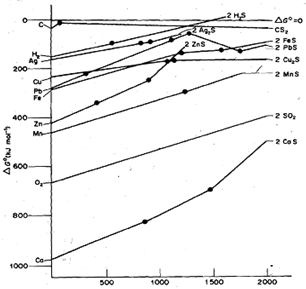
Figure shows the Ellingham diagram for sulphides of various elements. You can see from the diagram that carbon and hydrogen are not effective reducing agents for metal sulphides. Therefore, sulphides are first roasted in air to convert them to oxides, which are then reduced.
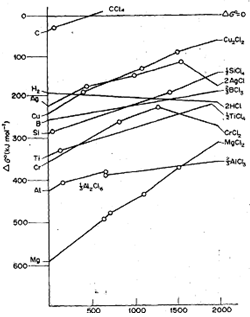
The Ellingham diagram for chlorides is shown In Fig. 1t can be concluded from the diagram that carbon is useless as a reductant for chlorides, but hydrogen can be used for his
purpose, specially at higher temperatures.