Q. Extraction of Iron oxide and titanium dioxide?
Iron oxide and titanium dioxide do not dissolve in the alkali and are filtered off as sludge. The solution is cooled and most of the aluminium hydroxide is precipitated either by the passage of carbon dioxide or by seeding with some+ freshly precipitated aluminium hydroxide:
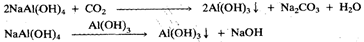
The silicates remain in solution, since silica is a more acidic oxide than alumina Aluminium hydroxide is filtered, washed and heated to give pure alumina.
2Al (OH) 3-------------------> Al2O3+ 3H2O
Alumina is dissolved in fused cryolite to which calcium fluorine is added to lower the melting point. The solution is then electrolysed at 1100-1300 K in an iron cell, lined with graphite, which acts as the cathode and carbon rods suspended in the electrolyte acting as the anode (Fig. 6.1). Electrolysis of the solution gives aluminium at the cathode and oxygen at the anode. The discharged aluminium sinks to the bottom of the cell and is tapped off. Fresh alumina is added as required. The anode is slowly attacked by liberated oxygen to form carbon monoxide. Therefore, anode has to be continually replaced, adding substantially to the cost of the process. The temperature of the cell is maintained by the passage of electric current. Following reactions take place during electrolysis:
Al2O3----------------------> 2Al3+ + 3O2-
3O2-----------------------> 3/2 2 + 6e at anode
2AL3+ 6e----------------> 2Al at cathode
Gallium, indium and thallium are usually obtained by electrolysing aqueous solutions of their salts. This method is not applicable in the case of aluminium salts as they are hydrolysed considerably by water.