Explain the discovery of neutron. Mention its properties.
Discovery of Neutron: The existence of neutron was first predicted by Rutherford in the year 1920. But it was discovered by Chadwick in 1932. In 1930, Bothe and Becker, two German scientists observed that a highly penetrating radiation was emitted when Boron or Beryllium were bombarded with particles of energy about 5 MeV emitted from polonium (P0 ) .These were thought to be high energetic g -rays because these are not affected by electric or magnetic fields.
4Be9 +2He4→[6C13]→6C13 +g
In this process, the total energy available is 14MeV which must be shared by the 136 C and g -photon. Hence their energy should be slightly less than 14MeV. Absorption measurements estimated that the g -photon energy should be about 7MeV. Curie and Joliot observed that when this radiation is passed through hydrogenated materials like paraffin, water, etc., high energy protons were ejected with a maximum energy of about 7.5MeV. From the calculation, it has shown that ejection of 7.5MeV protons needed g - photons as estimated by the measurement of the energy of protons expelled from paraffin is far below the value of 64MeV. Besides this, the energy of the radiation coming out from beryllium was predicted to be different, when the same radiation was incident on other hydrogenous substances. In other words, the energy of the same radiation was found to be having different values, when incident of different hydrogenous substances.
Thus this led to controversies about the energy of the g -photons.
Late in the year 1932, J.Chadwick observed concluded that these are a group of neutral particles of mass equal to that of protons. These neutral particles are called neutrons.
4Be9 +2He4→[6C13]→6C12+0n1+Q(Q= energy)
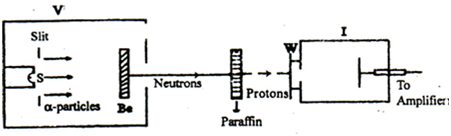
Figure shows the schematic diagram of Chadwick's experiment; g -particles were emitted from the sources S (polonium). These g -rays were allowed to bombard the Beryllium layer. The particles (Neutrons) coming out from the Beryllium were allowed to be incident on a paraffin block (w). It emits high energy protons which were then allowed to pass through an ionization chamber. Thus the neutron was discovered by Chadwick.
Properties of Neutron:
a) Neutron is an uncharged particle and hence it is not deflected by electric and magnetic field.
b) It was very high penetrating power and has ver low ionization power.
c) A free neutrons is unstable and spontaneously decays into a proton, electron and an antineutrino
d) If fast neutrons pass through substances like heavy water, paraffin wax, graphite etc. they are slowed down.
e) Neutrons are diffracted by crystals.