Q. Explain about Smelting process?
The roasted ore, which is generally an oxide, is strongly heated with a suitable reducing agent as result of which the metal is acquired in a molten state. This process is known smelting. In smelting a suitable chemical substance known flux is also added. The flux reacts with the gangue that remains after concentration to form a low melting compound known slag. He liquid metal and the liquid slag are immiscible and are simply separated. Generally the slag is lighter than the liquid metal and will be easily skimmed off from the surface of the molten metal. The gangue usually contains either basic oxides like CaO, FeO, etc., or an a acidic oxide like silica. When the gangue has a basic oxide, the flux used is an acidic oxide like silica: For gangues containing an acidic oxide, a basic flux likes CaO, FeO or lime stone is added.
SiO2+ CaO ----------------> CaSiO3
SiO2+ FeO----------------> FeSiO3
You have studied under roasting that HgO will be reduced to mercury by simply heating it to 800K - a temperature which maybe conveniently managed. Most oxides may be reduced to free metals by thermal decomposition at very high temperatures, but then the method becomes very expensive. Thus, by using a suitable reducing agent, reduction of metal oxides may be achieved at much lower temperatures. The choice of a reducing agent is guided by two considerations. First the reducing agent could be able to produce the desired metal at a low temperature. The second consideration is the cost of the reducing agent could be less expensive than the metal to be produced. Carbon in the form of coke is the least expensive reducing agent. Iron, tin, zinc, lead, cadmium, nickel, antimony, cobalt, molybdenum and many other metals are produced by carbon reduction of their oxides at temperatures up to 1800K. For example, zinc oxide is reduced to zinc:
ZnO (s) + C (s) ---------------> Zn(g) + CO(g)
Though, the reactions that occur in a high temperature carbon reduction process are not as simple as shown above. In most cases, the effective reducing agent is carbon monoxide, not carbon. This is due to both the metal oxide and cokes are solids, thus, contact between them is poor and direct reaction is slow:
MO(s) + C(s) -----------> M (l) + CO (g)
Though; carbon monoxide, which is a gas, makes a better contact with the solid metal oxide and the reaction proceeds more readily:
2C(s) + O2 (g) ----------> 2CO (g)
MO(s) + CO (g) -----------> M (l) + CO2 (g)
Some metals such as Cr, W, Mo, Ti, Mn, Mg, Al, etc., will be produced theoretically by reduction of their oxides with carbon, but they react with carbon to give metallic carbides. Thus, reduction with carbon is not a satisfactory process for producing these metals in a pure form. Hydrogen, though more expensive than carbon, is taken as a reductant for extraction of some of these metals, e.g., Ge, Mo and W:
GeO2 + 2H2 -----------> Ge+ 2H2O
MoO3+ 3H2----------> Mo + 3H2O
WO3+ 3H2----------> W + 3H2O
Though, many metals combine with hydrogen also to form metal hydrides. Thus, hydrogen also will not be used for the reduction of compounds of such metals. Highly reactive metals like Na, Ca, Mg and Al are used to displace these metals from their halides or oxides. These reactive metals are comparatively more expensive reducing agents due to they themselves are difficult or costly to prepare. The reduction of an oxide by aluminium is known as Goldschmidt's aluminothermic process.
Cr2O3(s) + 2Al(s) ------------------> 2Cr (l) + Al2O3 (l)
3MnO2 (s) + 4Al(s) ----------------> 3Mn (l) + 2Al2O3 (l)
3BaO(s) + 2Al(s) -------------------> 3Ba (l) + Al2O2 (l)
The reactions are extremely exothermic producing metals in the molten state. Other oxides commercially reduced by metals include UO3 (by Al or Ca), V2O5, MoO3 and W03 (by Al), Sc203, La2O3, ThO2 (by Ca) and Ta2Os (by Na).
Some metals will be more conveniently produced by reduction of their halides such as TiCl4,
ZrCl4, HfCb4, UF4, LaCl3, etc. by Mg, Ca or Na. This process is called as Kroll's process.
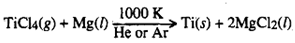
The most reactive metals, which will not be reduced by any other reducing agent, are prepared by electrolytic reduction of their compounds in molten state. Sodium, lithium, magnesium and aluminium are produced by this process. These metals are too reactive to be liberated by electrolysis of an aqueous solution.