Example of change of enthalpy:
Q 0.5kg/s of a fluid flows in steady state process. The properties of fluid at the entrance are measured as p1 = 1.4bar, density = 2.5kg/m3, u1 = 920Kj/kg while at exit properties are p2 = 5.6 bar, density = 5 kg/m3, u2 = 720Kj/kg. The velocity at entrance is 200m/sec, while at exit it is 180m/sec. It rejects 60kw of heat and rises through 60m during the flow. Find change of enthalpy and rate of work done.
Sol: Given that:
mf = 0.5kg/s
P1 = 1.4bar, density = 2.5kg/m3,
u1 = 920Kj/kg
P2 = 5.6 bar, density = 5 kg/m3,
u2 = 720Kj/kg. V1 = 200m/sec
V2 = 180m/sec Q = - 60kw
Z2 - Z1 = 60m h = ?
WS = ?
Since h2 - h1 = U + P
h2 - h1 = [U2 - U1 + (P2/ 2 - P1/ 1)]
= [(720 -920) × 103 + (5.6/5 - 1.4/2.5) × 105]
= [-200 × 103 + 0.56 × 105] = - 144KJ/kg
H = mf × (h2 - h1) = 0.5 × (-144) Kj/kg = -72KJ/sec .......ANS
By Applying SFEE
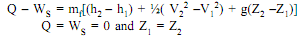
60 × 103 - WS = 0.5[ - 144 × 103 + (1802 - 1002)/2 + 9.81 × 60]
WS = 13605.7 W = 136.1KW .......ANS