Evaluation the Enthalpy of Steam:
Let
hf = Heat of liquid or sensible heat of water in KJ/kg
hfg = Latent heat of vaporization of steam in KJ/kg
ts = Saturation in 0ºC corresponding to given pressure.
tsup = Temperature of superheated steam in ºC
x = dryness fraction of wet saturated steam
Cp = Specific Heat of superheated steam at constant pressure in KJ/kg.k.
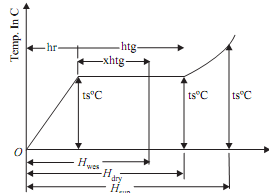
(a) Enthalpy of dry saturated steam
1 kg of water will be raised first for saturation of temperature (ts) for which hf (sensible heat of water) quantity of heat will be needed. Then 1 kg of water at saturation temperature will be transformed into 1 kg of dry saturated steam for which hfg (latent heat of steam) will be required. Thus enthalpy of dry saturated steam can be given by
Hdry (or hg)= hf + hfg kJ/kg
(b) Enthalpy of wet saturated steam
1 kg of water will be raised first to the saturation temperature (ts) for which hf (sensible heat of water) will be needed. Then 'x' kg of water at saturation temperature will be transformed into 'x' kg of dry saturated steam at same temperature for which x.hfg amount of heat will be required. Thus enthalpy of wet saturated steam can be given by
Hwet = hf + x.hfg kJ/kg
(c) Enthalpy of superheated steam
1 kg of water will be raised first to saturation temperature (ts) for which hf (sensible heat of water) will be needed. Then, 1kg of water at saturation temperature will be transformed into 1kg dry saturated steam at same temperature for which hfg (latent heat of steam) will be needed. Finally, 1kg dry saturated steam will be transformed into 1kg superheated steam at same pressure for which heat needed is
1 × Cp(tsup - ts) = Cp (tsup - ts) kJ
Thus enthalpy of superheated steam can be given by
Hsup = hf + hfg + Cp (tsup - ts) kJ/kg