Estimate total dissolved solids, Alkalinity and Hardness
A sample of water from Brazos River at Brenham was collected and analyzed for water quality characteristics and the data is presented in the table below (regular font). Estimate (i) total dissolved solids. (ii) Alkalinity (iii) hardness (TH, CH and NCH)
(Data is shown in Columns 1 and 2 other columns are part of solution)
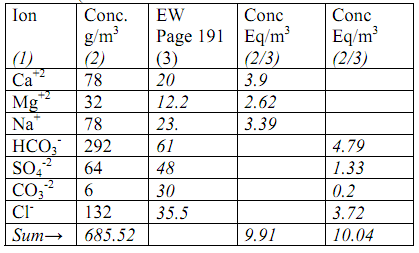
(i) Note that the sum of cations and anions should be roughly equal (in eq/m3) for a satisfactory analysis.
Total dissolved solids (TDS) = sum of all the ions in column 2 = 685.52 g/m3
(ii) Alkalinity is caused by the presence of mainly CO3-2 and HCO3 - ions (For all practical purposes the contribution of hydroxyl (OH-) and Hydrogen (H+) ions to alkalinity is negligible for natural waters)
Alkalinity (eq/m3) = CO32- + HCO3- = 4.79 + 0.2 = 4.99 eq/m3
(also remember that 1 eq/m3 = 50 mg/l as CaCO3)
(iii) Total Hardness, TH (add all the cations with a charge of 2 or more)
TH = Ca+2 + Mg+2 =
3.9 + 2.62 = 6.52 eq/m3 (or 326 mg/l as CaCO3)
Carbonate Hardness (CH) = bicarbonate + carbonate (However, CH cannot be more than TH)
= 4.79 +0.2 = 4.99 eq/m3 (250 mg/l as CaCO3)
Non-Carbonate Hardness (NCH) = TH - CH = 6.52-4.99 = 1.53 eq/3 (To obtain values in mg/L as CaCO3 multiply eq/m3 with 50 or use the column with mg/L as CaCO3).