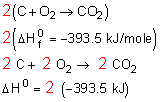Q. Calculate Enthalpy Changes in system?
Ans. It turns out that you can add reactions the same way you add algebraic equations. The enthalpy of the resulting reaction is the sum of the enthalpies of the added reactions.
Germaine Henri Hess, a Swiss chemist, was the first to figure out this principle. He said that when reactants are converted to products, the total enthalpy change is the same independent of whether the change occurs over one step or several steps.
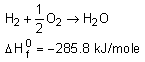
If the change happens over several steps we can use chemical equation algebra to calculate the change in enthalpy over the whole equation.
Here are things to keep in mind:
1) Flip equations.
Let's say you have written an equation with water on the reactants side:
But the final reaction involves water on the products side!
Just flip the equation around and change the sign of the enthalpy.
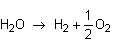
2) Multiply through by a coefficient.
Let's say you have written an equation with one mole of carbon dioxide on the products side

But in the final reaction there are 2 moles of carbon dioxide as the product!
Multiply everything through by 2.