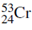Q. Determine Atomic Number and Mass Number?
One of the four naturally taking place isotopes of chromium has a mass number of 53. Determine the number of electrons, protons and neutrons in an atom of this isotope and write its symbol.
Begin by obtaining information on chromium from the periodic table. Chromium (Cr) is originate in the fourth row of the table in group 6 and the atomic number of chromium is 24.
You recognize that the atomic number of an element gives the number of protons in the nucleus of the atom.
Atomic number = number of protons = 24
You have as well learned that a neutral atom has equal numbers of protons and electrons consequently you can write the following expression.
Number of electrons = number of protons= 24
You as well know that the number of neutrons can be determined from the mass number and the atomic number.
Number of neutrons = mass number - number of protons
Number of neutrons = 53- 24= 29
To write down the symbol of this isotope follow this pattern.
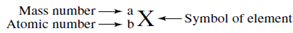
Substituting the known values provides this symbol for the isotope.