Detection and Handling of Gas Leakage : If you are using a toxic gas or have a store for it you must have some devices to indicate the leakage, if any. Though numerous monitoring instruments are available for this purpose, gas leaks may be indicated by formation of bubbles on pouring a dilute solution of soap on the suspected point of leakage.
However for few gases the following simple chemical procedures are also quite useful.
The source of leakage in a chlorine cylinder can be located by the use of ammonia. You may take a glass rod/stick and wrap a strip of filter paper/cloth on one end of it and soak into liquid ammonia or ammonium hydroxide. Take this chlorine detection device to a possible leakage point. Formation of dense white clouds (due to reaction of ammonia and chlorine) indicate the leakage source. Similarly hydrogen sulphide gas (H2S) can be detected simply by hanging strips of lead acetate paper near the place of work. Blackening of paper indicates leakage. You may be aware that in the chemistry laboratory H2S gas is used for the precipitation of captions of analytical groups I1 and IV in quantitative analysis.
Most of the times the leakage can be managed by simply tightening the valve or by fixing the delivery tube properly. You should attempt it only if there is no risk of exposure. If the leakage from the valve persists or leaks appear at any other position of the cylinder put on a gas mask and immediately remove the cylinder to open place and evacuate people from the vicinity. The leaks of chlorine, ammonia or hydrogen sulphide can be managed by passing the leaking gas into water fed scrubbing tower or by simply arranging to pass the gas through a column of water. Leaking oxygen poses a fire hazard if the concentration becomes 3-4% more than the normal, while hydrogen being flammable poses an explosion risk. Removing the cylinder to well ventilated area and ensuring the absence of ignition sources is sufficient. Leaking nitrogen and carbon dioxide cylinders should be end to open spaces to avoid exposure, else these do not pose much of threat. However, all leakages eventually should be managed by the experts.
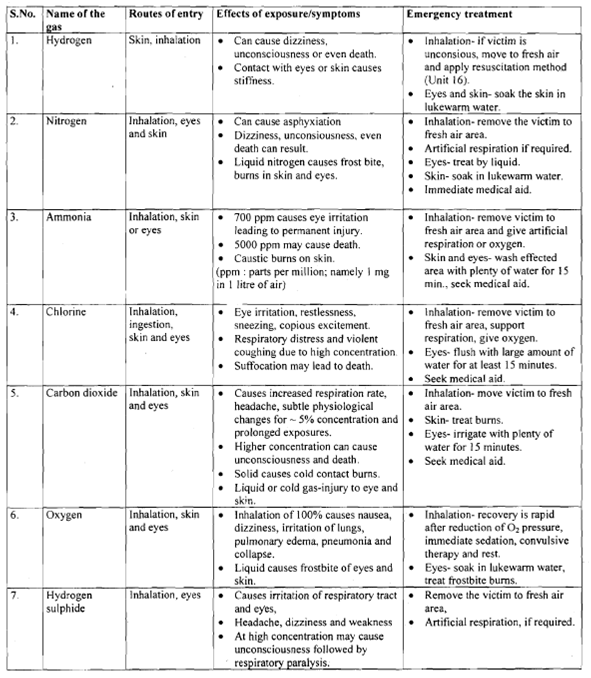
Exposure to the leaking gas may be inevitable despite the precautions taken. Therefore, a knowledge about the specific health hazards from the gases and their management, in case of an exposure, is essential. The health hazard data of some commonly used gases is summarised in table.
Several times, we need to evacuate glass apparatus in the Lab, This creates a low pressure inside the apparatus. These apparatus under low pressure are also potentially hazardous. Let us see in the next subsection how do we minimise this risk.