Q. Describe Water and Heavy Water?
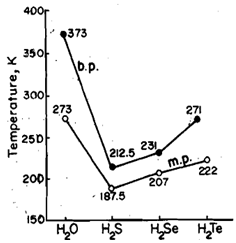
Water, H2O, the simple hydride of oxygen is a unique compound. It has extraordinarily high melting and boiling points compared to the other hydrides of the group, Fig. This is attributed to the high polarity of the molecule due to the large electronegativity difference between oxygen and hydrogen atoms. The high polarity of the molecule leads to very extensive association in water due to hydrogen bonding.
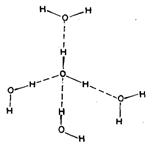
Polarity of water is responsible also for it being a very good solvent, especially for ionic compounds. Most reactions in nature take place in aqueous solutions, including the very complex reactions in the living systems. Ice, the solid state of water is a giant polymeric -molecule formed by tetrahedrally placed water molecules, held together by hydrogen bonds. Each oxygen atoms is surrounded by four hydrogen atoms. Two covalently linked and two hydrogen bonded. In turn, each hydrogen atom is surrounded by four oxygen atoms. Because of this arrangement of water molecules, ice has a cage structure with lot of empty spaces. It is lighter than liquid water. Ice, in fact, floats on the surface of water. The density of water varies with temperature and is maximum at 277 K. These two factors in a unique way are related with the sustenance of aquatic life. The ice once formed protects lower layers of water from further cooling, and the highest density at 277 K ensures that water at the lower depths will never get cooler than that. So, pou can see that in a way water has
Made the earth as we know it Its geographical features have been shaped by various processes in which water is an active agent. The living beings also owe their existence to water.
You know that, if the hydrogen atoms in water molecule are replaced by deuterium, an isotope of hydrogen with mass number= 2, then we get what is called heavy water or D20. Melting point, 276.81 K, boiling point, 374.42 K, density, 1, 1044 x 103 kg m-3and viscosity, 1.107 centipoise, of D20 are higher than those of H20. This is attributed to higher molecular weight of D20,
Water is the only practical source of deuterium where it is present to the extent of 0.0145%. D2O can be produced by electrolysis or repeated fractional distillation of water. These two processes are quite expensive as a large amount of energy is required for these processes. Heavy water is used on a large scale as a moderator in nuclear reactors.