Q. Describe about Electron Configurations?
As you will study the number and arrangement of electrons around the nucleus of an atom determines its chemical properties. Consequently it is useful to be able to determine and write out the electron arrangement called an electron configuration of an atom in symbolic form.
The Writing electron configurations the electron configuration of an atom is written by stating the number of electrons in every energy sublevel and writing the sublevels in order of increasing energy. The numerous electrons in an energy sublevel are indicated by a superscript integer. For instance the electron configuration of a hydrogen atom which has one electron is written as 1s1. This specify that hydrogen's one electron is in the 1s sublevel.
Helium has two electrons. Recollect that an s sublevel consists of a single orbital that can hold a maximum of two electrons. Consequently helium's second electron fills the one available orbital in the 1s sublevel and its electron configuration is written as 1s2.
A neutral lithium atom has three electrons. The primary principal energy level is filled with two electrons. Where does the third electron go? This electron is discovered in the second principal energy level which like all energy levels begins with an s sublevel. Consequently lithium has two electrons in the 1s orbital and a third electron in the 2s orbital giving it the electron configuration 1s22s1. With the beryllium which has four electrons the 2s orbital is filled with two electrons yielding a configuration of 1s22s2.
Element boron has five electrons four of which drop into the same configuration as beryllium. Evoke that the second principal energy level has two energy sublevels available (s and p) and that the p sublevel is of higher energy than the s sublevel. One regulation governing electron configurations is the aufbau principle which states that every successive electron occupies the lowest energy orbital available. In the circumstances of boron the lower-energy s orbitals are full so the fifth electron is found in one of the three available orbitals in the higher-energy 2p sublevel. Consequently boron's electron configuration is 1s22s22p1.
Evoke that the three orbitals available in a p sublevel can each hold a maximum of two electrons. Progressing from boron during carbon (1s22s22p2) nitrogen (1s22s22p3) oxygen (1s22s22p4) fluorine (1s22s22p5) and neon (1s22s22p6) the 2p sublevel becomes filled with six electrons.
With the element sodium the eleventh electron starts the 3s sublevel to give the configuration 1s22s22p63s1. The same pattern that take place with lithium through neon repeats here with successive electrons filling the 3s orbital and 3p orbitals. The element argon has a filled 3p sublevel. Argon has 18 sum electrons and the configuration 1s22s22p63s23p6.
You may evoke that three sublevels-s, p and d-are available in the third energy level so you might expect the next electron to begin the 3d sublevel. Nevertheless a complication take place here because the 4s sublevel is of lower energy than the 3d sublevel. Therefore following the aufbau principle the next (nineteenth) electron begins the 4s sublevel in the element potassium producing the configuration 1s22s22p63s23p64s1. Scandium is the primary element that has electrons in the 3d sublevel. As an instance of an element that has electrons in the 4p sublevel the electron configuration of arsenic is 1s22s22p63s23p64s23d104p3. Note that the configuration is written by placing the sublevels in order of increasing energy not in numerical order.
To place energy sublevels in order of increasing energy, it is useful to learn the following energy sublevel diagram. By following the arrows, you can write the energy sublevels in the correct order.
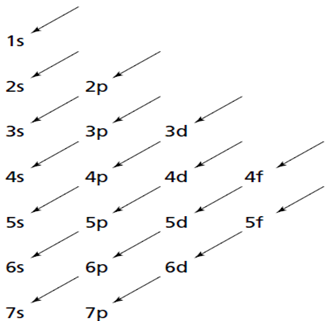
For the reason that a new principal energy level always begins with the element immediately following one of the noble gases it is possible to simplify electron configurations by using the symbol of the previous noble gas to denote all of an atom's inner-level electrons. As a easy example consider nitrogen's electron configuration of 1s22s22p3.
Helium the preceding noble gas has the configuration 1s2. Therefore the symbol [He] can be substituted into nitrogen's configuration to give [He] 2s12p3. This type of an electron configuration is called noble-gas notation.