Define Proteins as Enzymes?
From conception to death, living cells use oxygen and metabolize fuel. Cells synthesize new products, degrade others, and generally are in a state of metabolic flux. For these processes to occur, catalysts are needed to enhance each of the many thousands of reactions occulring in the cell. These catalysts called 'enzyrnes' are proteins. Enzymes make up the largest and the most specialized class of proteins. Each enzyme is unique and catalyzes a specific kind of reaction. In the cell, enzymes are found in cellular compartments (cytoplasm, nucleus, mitochondria, etc.), as well as, the membranes within and around the cell wall. The location of an enzyme is one of its characteristics and dictates, in part, its role in metabolism. Many enzymes are complex proteins; they consist of a protein component and a prosthetic group. The protein part is called apoenzyme and the prosthetic group, 'coenzyme' as illustrated in Figure
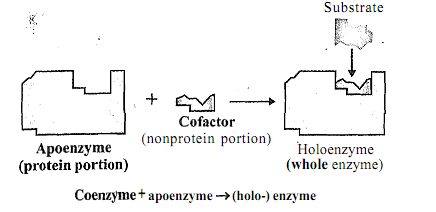
Figure: Holoenzyme
Enzymes consist of specific sequences of amino acids. The catalytic function of an enzyme is intimately related to its amino acid sequence. Enzymes must possess a shape that will complement the reactive molecular shape of the substrate in the same way as a key fits into a lock. This is commonly referred to as 'lock and key mechanism'. This shape is a function of the enzyme protein's primary, secondary, tertiary and quaternary structure. In the same way, substrates should also have specific shapes in order to be catalyzed by their respective enzymes.
This is the reason why only D-sugars or L-amino acids can be metabolized by mammalian cells. These stereoisomers conform to the shape required by the enzyme which serves as its catalyst. While enzymes show absolute specificity, the specificity generally applies to the entire molecule. If however the substrate is large and complex, the structural requirements are less stringent in that only that part of the substrate involved in the enzyme-substrate complex should have the appropriate molecular arrangement. The portion of the substrate not involved in the reaction need not be the appropriate conformation. Some enzymes are specific for only one substrate; others may catalyze several related reactions. While some are specific for a particular substrate, others are specific for certain bonds. This is called 'group specificity'. For example, glycosidases act on glycosides, pepsin and trypsin act on peptide bonds and esterases act on ester linkages. Within this group, certain enzymes exhibit greater specificity. Chymotrypsin preferentially acts on peptide bonds in which the carboxyl group is a part of the aromatic amino acids (phenylalanine, tyrosine or tryptophan). Enzymes such as carboxypeptidase catalyzk the hydrolysis of the carboxy-terminal or amino-terminal amino acid of a polypeptide chain. This bond specificity, rather than molecular specificity, is useful to the animal in that it reduces the number of enzymes needed within the organism. Incidentally, the above enzymes are very useful to the protein chemist in his 1 her determination of the amino acid sequence of a given protein. Cells synthesize enzymes in much the same fashion as they synthesize other proteins; yet enzymes are relatively short lived. Cells must continually synthesize their enzymes if they are to survive. So, we have looked at the functions of proteins as enzymes. Proteins also function as carriers in the body.