Combustion process:
Air from the engine compressor enters the combustion chamber at a velocity up to 500 feet per second, but because at this velocity the air speed is far too high for combustion, the first thing that the chamber must do is to diffuse it, i.e. decelerate it and raise its static pressure. Because the speed of burning kerosene at normal mixture ratios is only a few feet per second, any fuel lit even in the diffused air stream, which now has a velocity of about 80 feet per second, would be blown away. A region of low axial velocity has therefore to be created in the chamber, so that the flame will remain alight throughout the range of engine operating conditions.
In normal operation, the overall air/fuel ratio of a combustion chamber can vary between 45:1 and 130:1. Kerosene, however, will only burn efficiently at, or close to, a ratio of 15:1, so the fuel must be burned with only part of the air entering the chamber, in what is called a primary combustion zone. This is achieved by means of a flame tube (combustion liner) that has various devices for metering the airflow distribution along the chamber.
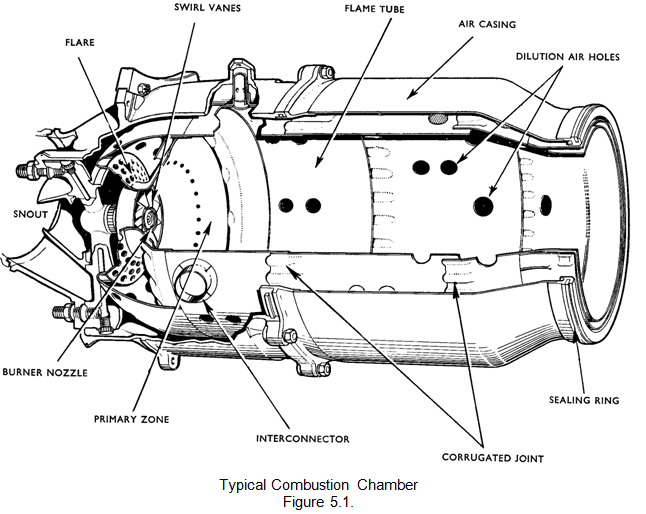
Approximately 20 per cent of the air mass flow is taken in by the snout or entry section. Immediately downstream of the snout are swirl vanes and a perforated flare, through which air passes into the primary combustion zone. The swirling air induces a flow upstream of the centre of the flame tube and promotes the desired recirculation. The air not picked up by the snout flows into the annular space between the flame tube and the air casing.
Through the wall of the flame tube body, adjacent to the combustion zone, are a selected number of holes through which a further 20 per cent of the main flow of air passes into the primary zone. The air from the swirl vanes and that from the primary air holes interacts and creates a region of low velocity recirculation. This takes the form of a toroidal vortex similar to a smoke ring, and has the effect of stabilising and anchoring the flame. The recirculating gases hasten the burning of freshly injected fuel droplets by rapidly bringing them to ignition temperature.