Chemical action in lead acid battery:
When the lead acid battery is delivering current, the sulphuric acid breaks up into Hydrogen ions (H2) carrying a positive charge and Sulphate ions (SO4) carrying a negative charge. The SO4 ions combine with the lead plate (Pb) and form lead sulphate (PbSO4). At the same time they give up their negative charge, thus creating an excess of electrons on the negative plate.
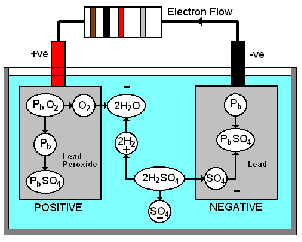
The H2 ions go to the positive plate and combine with the oxygen of the lead peroxide (PbO2) forming water (H2O), during the process they take electrons from the positive plate. The lead of the lead peroxide combines with some of the SO4 ions to form lead sulphate on the positive plate.
The result of this action is a deficiency of electrons on the positive plate and an excess of electrons on the negative plate.
When a circuit is connected to the battery, electrons flow from the negative plate to the positive plate. This process will continue until both plates are coated with lead sulphate. The lead sulphate is highly resistive, and it is mainly the formation of the lead sulphate which gradually lowers the battery capacity until it is discharged.
During charging, current is passed through the battery in a reverse direction. The SO4 ions are driven back into solution in the electrolyte, where they combine with the H2 ions of the water, thus forming sulphuric acid. The plates are thus returned to their original compositions.
The sulphuric acid is effectively used up as the battery is discharged, and returned to the electrolyte as it is charged, a test of the specific gravity of the electrolyte will give a good indication of the state of charge of the battery.