Calculate change of entropy of universe:
5Kg of ice at 100C is kept in atmosphere that is at 300C. Calculate change of entropy of universe when it melts and comes into thermal equilibrium with atmosphere. Take latent heat of fusion as 335KJ/kg and specific Heat of ice is half of that of water.
Sol: Mass of ice, m = 5Kg
Temperature of ice = -100C = 263K Temperature of atmosphere = 300C = 303K
Heat absorbed by ice from the atmosphere = Heat in solid phase + latent heat + heat in liquid phase
= miCidT + MiLi + mwCwdT
= 5 × 4.187/2 ( 0 + 10) + 5 × 335 + 5 × 4.187 × ( 30 - 0)
= 104.675 + 1675 + 628.05
Q = 2407.725KJ
Entropy change of atmosphere ( ? s)atm = - Q/T = -2407.725/303
(?s)atm = - 7.946KJ/k
Entropy change of ice(?s)ice
= Entropy change as ice gets heated from - 100C to 00C + Entropy change as ice melts at 00C to water at 00C + Entropy change of water as it gets heated from 00C to 300C
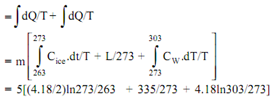
= 5 x 1.7409 = 8.705KJ
Entropy of universe = Entropy change of atmosphere (?s)atm + Entropy change of ice( ? s)ice
= - 7.946KJ/k + 8.705KJ
= 0.7605329KJ/kg .......ANS