Reference no: EM132951398
1) Which compounds will give a positive test with Tollens's reagent (silver mirror)?
I. 2-pentanone II. Pentane III. 1-pentanol IV. ethyl methyl ether V) propanal
A) I and lV only B) I and II only C) I, II, and III only D) I, II, III, and IV E) V only
2) Which compounds will not give a poisitive test with Tollens's reagents?
I. CH3CH2-O-C(CH3)3 II.CH3CH2CH2CH2CH2CHO
III.CH3CH2CH2CH2CH2CH2OH
IV. 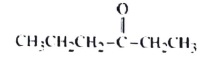
A)II and IV only B) I and only C) II and III only D) I, II III, and IV E)I III,and lV
3) Which compound is an acetal?
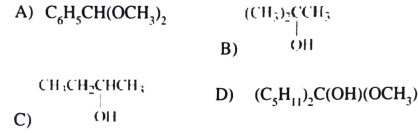
4) Which of the above are hemiacetals?
5) Which compound undergoes reduction with H2/Pt (or LiAlH4 followed by water)?
A) CH3(CH2)3OCH3 C) CH3(CH2)3,CH2OH
B) CH3(CH2)3CHO D) CH3(CH2)3CH3
5 b)Write the product of the reduction of the above compound.
6) Which compound does not undergo reduction with hydrogen and platinum?
A) 2-pentanone C) 2-pentanol
B) 2-hydroxypentanal D) 2-butene
6b)Write the products of the reduction of the compounds that undergo reduction.
7) Write the name of the carboxylic acid derivative (ester, amide etc.)
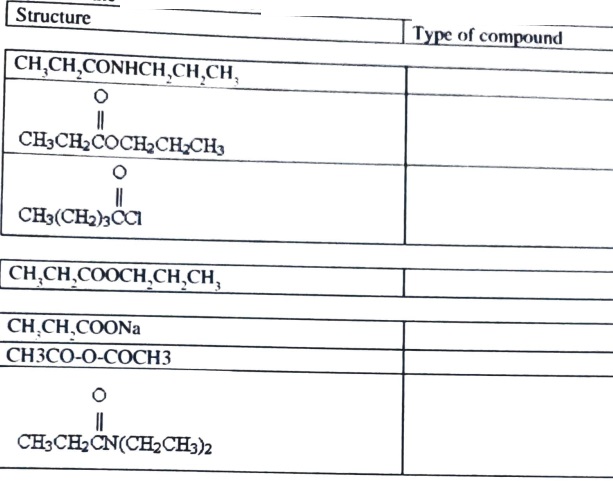
8) Treatment of 1-hexanol with potassium permanganate (or potassium dichromote)will yield:
A) only hexanal B) 1-hexanone C) 2-hexanone D) primarily hexonoic acid
9) Treatment of 2-pentanol with potassium permangannte will yield:
A) only pentanal B) I -pentanone C) 2-pentanone D) primarily pentanoic acid
10) The IUPAC and common names of this compound are, respectively:
CH3COOH
A) ethanoic acid acid and formic C) formic acrid and ethanoic acid
B) ethanoic acid and acetic acid D) acetic acid and ethanoic acid
11) Which compound is a soap?
A) CH3(CH2)2COONa C) CH3(CH2)14COONa
B) CH3(CH2)2,COOH D) CH3(CH2)14COOH
12) Whlch of these compounds are amines?
I. CH3CH2NHCH3 II. CH3CH2CONHCH3
III. C6H5NO2 lV. (CH3)2NCH2CH3
A) I, II, and IV only B) 1 and II only C) II and III only D) 1 and lV only
13) Mark as primary, secondary, amines or not an amine
I. CH3CH2NHCH3 II. CH3CH2CONHCH3
III. C6H5NH2 IV. (CH3)2NCH2CH3
14) Which of these compounds are amides?
I. CH3CH2NHCH3 III. C6H5NO2
II. CH3CH2CONHCH3 IV. (CH3)2NCH2H3
15) Which amine can hydrogen bond to itself?
I. ethylamine II. diethylami III. triethylamine
A) I and II only B) II and III only C) I and Ill only
16) Which amine cannot hydrogen bond to itself?
I. methylamine II. ethylmethylamine III. trimethylamine
A) I only B) II only C) III only D) I and II only
16 b)Which of these above amines can H bond to water?
16 c)Which of these above amines can H bond to water but not to itself?
17) Which statement about these compounds is true?
I. propylamine II. 1-propanol III. phenol
A) I will turn red litmus paper blue. C) III will turn red litmus paper blue.
B) II will turn red litmus paper blue. D) II will turn blue litmus paper red.
18). This compound is:
CH3(CH2)3CONHCH3
A) a secondary amine C) a primaryamide
B) a secondary amide D) a tertiary amide
19) Which compound does not react with an acid or acyl chloride (acid achloride) to produce an amide?
A) propylamine B) dimethylamine C) trimethylamine D) isopropylmethylamine
20) An amide dissolved in water is:
A) acidic
B) basic
C) neutral
D) basic if the amide is tertiary; acidic if the amide is primary or secondary
21) Write the product of the reaction between Proply react amine and acetic acid (CH3COOH) at 100°C
22) Write the product of hydrolys is of CH3CH2CONHCH3 iN
i) Acid medium ii) basic medium
23) Classify the following as acidic, basic, neutral
A) carboxylic acid
B) Amines
C) Amides
D) esters
E) Phenols
24) Choose the correct statement:
A) Pentanal has a much lower boiling point than that of 2-pentanone.
B) Pentanol is less soluble than 2-pentanone in water.
C) Pentanal is more soluble than pentue in water.
D) Pentanol and propanal have approximately the same boiling point
25) Which compound(s) can be synthesized by reduction of an aldehyde?
I. (CH3)3C-OH II. (CH3)CHCH2OH
III. IV. CH3(CH2)3CH3
A)I and II only B) II Only C) I and III Only D) II and IV only
26) Which compounds react to form this compound?
I.(CH3CH2)2C(OCH3)2 II. (CH3CH2)2CH-OH
(CH3CH2)2C=O
III. CH3OH IV. (CH3)2C= O
A) I and II B) I and III C) I and IV D) II and III
27) Arrange these compounds in order of decreasing melting point.
I. butanal II. propanoic acid III. potassium propanoate
A) I > II > III B) II > I > III C) I > III > II D) III > II > I
28) Arrange these compounds in order of decreasing boiling point.
I. CH3(CH2)4COOH II. CH3(CH2)5CH2OH III. CH3(CH2)5CHO
A) I > III > II B) II > I > III C) II > III > I D) I > II > III
29. What is the IUPAC name of this compound?
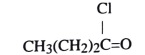
A. butyl chloride B. butanoic chloride
C) butanoyl chloride D) butanone chloride
30. What is the IUPAC name of this compound?
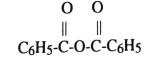
A) benzylic anhydride B) benzoic anhydride
C) benzokeher D) benzoic esler
31) Which statement is false?
A) Primary and secondary amines have melting points and boiling points comparable to those of aldehydes and ketones.
B) Tertiary amines have melting and boiling points considerably higher than those of primary arid secondary amines.
C) Tertiary amines have melting and boiling points comparable to those of ethers andhydrocarbons.
D) Amides have higher melting and boiling points than those of carboxylic acids.
32) What functional groups are present in this compound?
(CH3)2NCH2OCH2CON(CH3)2
a. a tertiary amine, an ether, and a tertiary amide
b. two tertiary amides,
c. an amine, an ether, and a secondary amide
d. two teniary amides and a ketone
33) An acid chloride and a secondary amine will produce:
a. A tertiary amide
b. A secondary amide
C. an amine hydrochloride
d. These compounds cannot react with one another.
34) What is the IUPAC name for this compound?
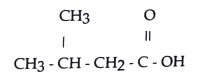
A) pentanoic acid
B) 2-methylbu tanoic acid
C) 3-methyIbutanoic acid D)2-methyl-4-butanoic acid
35) Write the structure of 2-ethyl-3- propy1-heptanoic acid
36) Which of the following is NOT an alkaloid (plant derived amine containing compounds with physiological effect:s)?
A) nicotine
B) caffeine
C) diethylamine
D) quinine
E) cocaine