Reference no: EM131334516
Question 1:
This is a tandem MS of a tryptic peptide. The mass of the intact peptide is 1569.692 Da.
a. There are two immonium ions labelled with their mass-to-charge ratio in the spectrum. Label the signals with their single-letter amino acid abbreviation.
b. The other peaks that are labelled are y-ions. That is they are fragments that all contain the C-terminus of the peptide. Using these m/z values and the residue masses in the table, determine as much of the sequence as you can. Write out the amino acid sequence from the N- to the C-terminus.
c. Determine the difference in mass of the intact peptide and the y-ion with the highest m/z.
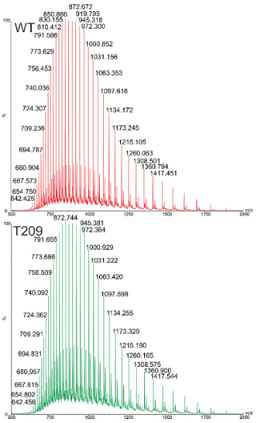
Question 2:
The mass spectra to the left show two proteins. One is the wild type version of a protein and the other is a mutant where threonine 209 has been replaced by another amino acid. By determining the mass of each protein and using the amino acid residue mass table, which amino acid has the threonine been replaced by?
Question 3:
Fructose bisphosphate (FBP) aldolase is an enzyme that catalyses the formation of FBP from glyceraldehyde-3-bisphosphate and dihydroxyacetone phosphate (DHAP).
The Class I FBP aldolase found in E. coli amongst other bacteria uses an active site lysine to form a Schiff's base with DHAP as part of the reaction mechanism. Mass spectrometry has been used to confirm the amino acid sequence of the FBP aldolase and to identify the position of the active site lysine.
a. The calculated mass of the FBP aldolase is 37,964.3 Da. The mass measured by mass spectrometry was found to be 37,979.3 Da. DNA sequencing of the cloned gene revealed 2 changes to the reported sequence: Val191 was replaced by Leu and Ile308 was replaced by Asn. Does the mass spectrometric data confirm these changes? Explain your answer.
Class I FBP aldolases are characterised by the formation of a Schiff's base intermediate with dihydroxyacetone phosphate (DHAP). This involves the reaction of an amine with a carbonyl to give a hydroxylamine which condenses to the imine, or Schiff's base. To trap the Schiff's base intermediate, it was reduced with sodium borohydride.
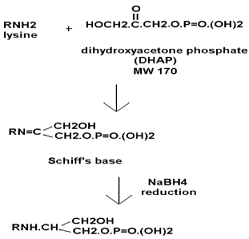
b. Calculate the expected, overall mass difference of the derivative product after sodium borohydride reduction. (The theoretical, average atomic masses are: C = 12.0110, H = 1.0079, O = 15.9994, P = 30.9738). Compare the final structure (bottom) with the initial protein structure (top, left) to calculate the change in mass.
The modified Class I aldolase was digested with a protease to give a mixture of peptides which were purified by HPLC. Mass measurements of the HPLC fractions indicated that the peptide containing residues 194 to 239 of the aldolase contained the expected modified lysine:
194- VT VLWAYLRNSA FKKDGVDYHV SADLTGOANH LAATIGADIV KQKM-239
This fragment has 4 lysine residues. Lysine 207 and lysine 208 were ruled out as being derivitised by using amino acid sequencing from the N-terminus. The N-terminal sequencing did not get as far as the other two lysines. Two mutants were created, one with K236A and the other with K238A, and their molecular masses were confirmed by mass spectrometry. Both were treated independently with DHAP/NaHBH4, and their masses measured again. The mass spectrometric data for the two mutants together with the wild-type aldolase are summarised in the Table:
|
Aldolase
|
Calculated mass
|
Measured mass
|
Mass after DHAP/NaBH4 treatment
|
|
Wild Type
|
37978.1
|
37979.3
|
38134.3
|
|
K236A
|
37921.1
|
37923.8
|
37921.9
|
|
K238A
|
37921.1
|
37923.5
|
38076.3
|
c. From the table above, which aldolase mutant reacts with DHAP?
d. Which lysine is responsible for enzyme activity, K236 or K238?
e. Suggest another method of determining which lysine is the active site in the fragment 194 -239. Include some mass spectrometry in your analysis.
Attachment:- Assignment.rar
|
Compare the net present value
: This question basically asks you to compare the Net Present Value (NPV) to the Internal Rate of return (IRR) on the time length of an investment project.
|
|
Nature of educational dualism in developing countries
: Describe the nature of educational dualism in developing countries and its implications for the character of growth. Also, elaborate on how the LDC educational system actually contributes to the reproduction of social inequality across generation..
|
|
Find the cost minimizing quantity of output
: A large number of firms selling homogenous (identical) products serve an industry. Each of the firms has the following cost structure: + 10Q + Q2, so that +2Q. The firm-level cost structure holds regardless of how many firms enter the industry. D..
|
|
Deposit each year to achieve your goal
: You want to have 1 million in real dollars in an account when you retire in 30 years. The nominal return on your investments 11 percent and the inflation rate is 3.8 percent. What real amount must you deposit each year to achieve your goal?
|
|
Write out the amino acid sequence from n- to the c-terminus
: The other peaks that are labelled are y-ions. That is they are fragments that all contain the C-terminus of the peptide. Using these m/z values and the residue masses in the table, determine as much of the sequence as you can. Write out the amino ..
|
|
Spectacular growth of investments in manufacturing
: Use the microeconomic theory of fertility to argue that it was a rise in wages, spectacular growth of investments in manufacturing technology etc., that played a key role in reducing family size and slowing population growth in East Asia.
|
|
More inventory will allow a retailer to fill orders promptly
: While keeping more inventory will allow a retailer to fill orders promptly, it may impede another retailer depending on the industry they are in. clothing can sit on a rack indefinitely, but food spoils and does not have a long shelf life. It is extr..
|
|
Determine the value of ps
: Determine the value of Ps if it is desired to expand the bandwidth of the system to 10 kHz, while maintaining the same SNR at the detector.
|
|
Management of the firm retail cash collection system
: As cash manager for an oil company, you are responsible for the firm’s cash management activities. One of these activities is the management of the firm’s retail cash collection system. Your firm receives an average of 130,000 remittances per month w..
|