Reference no: EM132054720
You should provide a word-processed solution using Word, and including relevant output from Excel by. Upload your files to the VLE.
Question 1.
A chemical reactor is operated in series with a storage tank as illustrated in Figure 1 below. The volume of the storage tank is V1 and the volume of the reactor is V2. The chemical reaction processes only happen in the presence of light and no light enters the storage tank.
The reaction process is A → B → C
The first reaction is first order with respect to A and has a rate constant k1 [min-1]. The second reaction is first order with respect to B and has a rate constant k2 [min-1]. Both reactions are irreversible and the density of the fluid remains constant. The storage tank and the reactor are instantaneously well mixed. The volumetric flow rate is q [litres/min] and the volume of the pipes connecting the storage tank and reactor can be neglected.
The concentrations of A, B and C in the storage tank are CA1, CB1 and CC1 moles per litre. The concentrations of A, B and C in the reactor are CA2, CB2 and CC2 moles per litre.
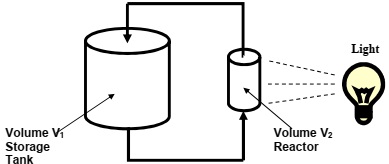
Figure 1: Configuration of Reactor and Storage Tank
(a) Write down a generalized material balance equation for a chemical reactor in terms of the words input, output, disappearance and accumulation.
(b) For the storage tank construct a table giving mathematical expressions for the four terms input, output, disappearance and accumulation for the chemical species A, B, and C and hence derive three equations for, respectively, the rates of change of the concentrations of A, B and C with time.
(c) For the reactor construct a table giving mathematical expressions for the four terms input, output, disappearance and accumulation for chemical species A, B and C and hence derive three equations for, respectively, the rates of change of the concentrations of A, B and C.
(d) Before the light is switched on fluid is circulated so that the contents of the storage tank and the reactor are uniform and only A is present. The differential equations you have derived are to be solved using a numerical approach: specify what the initial boundary conditions are for the equations that describe the storage tank and reactor.
(e) The rate constant k1 = 1.0 min-1 the initial concentration of A = 1.0 mol/litre. Consider the following cases:
(i) V1 = 95 litres, V2 = 5 litres, k2 = 0.2 min-1 volumetric flow rate is q = 1 litre/min.
(ii) V1 = 95 litres, V2 = 5 litres, k2 = 1.5 min-1 volumetric flow rate is q = 1 litre/min.
(iii) V1 = 50 litres, V2 = 50 litres, k2 = 0.2 min-1 volumetric flow rate is q = 10 litres/min.
(iv) V1 = 50 litres, V2 = 50 litres, k2 = 1.5 min-1 volumetric flow rate is q = 10 litres/min.
For each of the four cases use Excel or another suitable approach to produce chemical species concentration versus time plots by solving the differential equations you have derived using the boundary conditions you have specified. In each case produce one plot for the storage tank showing CA1, CB1 and CC1 versus time and one plot for the reactor showing CA2, CB2 and CC2 versus time. Comment on how the product distribution, the relative amounts of chemical species B and C produced, is affected by changing the relative volumes of the storage tank and reactor and on the effect of the value of the rate constant k2.
Question 2.
Before attempting this question you must send an email to and you will be instructed which set of solubility data to use from the Appendix.
2(a) Compare the experimentally determined, solubility data points for the specified solvent with those you determine from the ideal solubility equation showing all your working out. The solute has a molecular formula C15H12N2O, a melting point of 175°C and a molar heat of fusion of 26.8 kJ/mol. By what factor does the experimental solubility data differ from the predicted solubility and how does this factor change as a function of temperature? Comment on the differences you find.
2(b) Convert the experimental solubility data to units of grams of solute per litre of solution. To do this you will need to locate a source of data relating to the density of the specified solvent and provide a reference. The density of the solute in its solid form is 1.35 g/cm3. State your assumptions in performing these calculations.
2(c) Using Excel plot the solubility data in mol/litre versus saturation temperature.
2(d) Estimate the molar enthalpy of crystallization, ΔHcryct, of the solute in the specified solvent using the relation ΔHcryct = -ΔHdsol where ΔHdsol is the last differential molar heat of solution and dlnCeq/dT = ΔHdsol/RT2. Integrating ln(Ceq) = -ΔHdsol/RT.
2(e) Nucleation occurs at a supersaturation of 1.3. You start cooling a 1000 litre batch of solution from 60°C with a solute concentration equivalent to a saturated solution at 50°C. At what temperature does nucleation occur?
2(f) Assume the rate of mass transfer due to crystallisation from the solution phase to the solid phase is directly proportional to the level of supersaturation. The objective is to maintain a constant rate of mass transfer after nucleation until the level of supersaturation has been reduced to 1.05. If the batch crystallisation process is to take 3 hours, work out and explain how the temperature in the vessel should be changed as a function of time. What mass of crystals will be produced?
Attachment:- Question.rar