Reference no: EM13168960
Required
Write a report to the client (Geolab Exploration Limited) which covers the following;
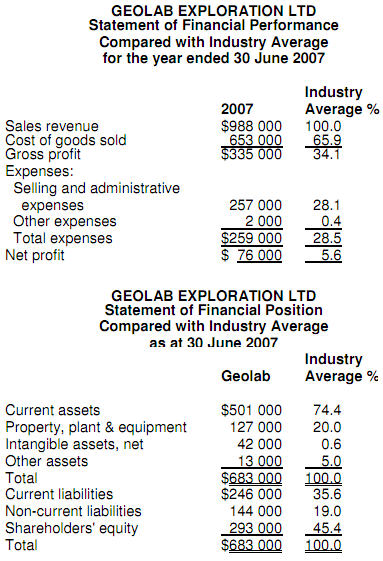
a. A two-column common-size statement of financial performance and statement of financial position for Geolab. The first column of each statement should present Geolab's common-size statement, and the second column should show the industry averages.
b. A profitability analysis. Calculate relevant ratios and identify strengths and weaknesses. Where there are weaknesses suggest solutions?
c. An analysis of short-term and long-term financial position. Calculate relevant ratios and identify strengths and weaknesses. Where there are weaknesses suggest solutions.
d. Advise Geolab about the financing their $200,000 requirement. Given their financial statement, what advise would you give to Geolab about how to raise this amount? Include in your report if they can be optimistic in raising this finance from a lender, options to raise the finance, pros and cons of each option, taxation implications and your recommendation about the terms of finance they should seek.
e. Lenders that are concerned about the ability of Geolab Limited to repay the loan, if advanced, together with the interest. Explain what Geolab can do to alleviate the lenders' worries
f. Limitations of the above analysis.