Reference no: EM132668246
CHT353 Drug Targets - Cardiff University
Beta-lactam antibiotics and beta-lactamases
Question 1. Meropenem is one of the new broad spectrum beta-lactam antibiotics that prevents bacterial cell wall synthesis. Although it is active against gram-positive bacteria, it exhibits better activity against gram-negative bacteria.
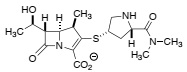
meropenem
Write a curly-arrow mechanism showing how meropenem inhibits its target enzyme, and explain (using a clear diagram) the molecular basis of the unusually high reactivity of the carbonyl group in the beta-lactam ring.
Question 2. Bacteria that express class B beta-lactamases, which contain a Zn(II) ion at the active site, are generally resistance to treatment with meropenem. Write down the reaction product that is formed when the hydrolysis of this antibiotic is catalysed by a class B beta-lactamase.
Question 3. Steady-state kinetic studies showed that kcat/KM = 0.9 μM-1s-1 for meropenem hydrolysis that is catalysed by the class B beta-lactamase expressed in Pseudomonas aeruginosa. State whether meropenem is a good substrate for this enzyme and briefly justify your answer.
Question 4. When treating bacterial infections caused by Pseudomonas aeruginosa, meropenem is usually co-administered in a pill that also contains vaborbactam (shown below).
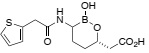
vaborbactam
Why is vaborbactam is included in the pill ? Write a curly-arrow mechanism for the interaction of vaborbactam with its drug target.
Other bacterial drug targets
Question 5. Cycloserine is a potent inhibitor of alanine racemase, which is a PLP-dependent enzyme. Using clear diagrams, briefly explain why inhibiting alanine racemase prevents cell wall biosynthesis.
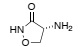
cycloserine
Question 6. Incubating alanine racemase with cycloserine yields an adduct with the following structure.
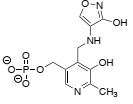
adduct
Given that the amino group of cycloserine mimics that of L-alanine in the active site of alanine racemase, write a curly-arrow mechanism that explains the formation of this adduct and propose a reason to explain why it is unusually stable.
Question 7. Linezolid is a relatively new class of antibiotic, which binds to the 50S subunit of bacterial ribosomes. Draw a cartoon showing the intermolecular interactions between linezolid and its binding site in the 50S subunit, and propose a reason why linezolid does not bind tightly to human ribosomes.
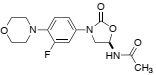
linezolid
Question 8. Does the fluorine substituent that is present in linezolid have a function? If so, then briefly explain why fluorination might be important for the properties of the drug.