Reference no: EM133104006
Question 1: (a) Artemisinin 2 is a natural product that can be obtained from artemisinic acid 1 by chemical synthesis, as shown below.
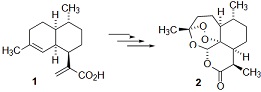
Identify the functional group in artemisinin 2 that is necessary for its biological activity and suggest why compound 2 might kill the parasites that cause malaria.
(b) Three enzymes (ADS, a cytochrome P450 and a cytochrome P450 reductase) were introduced into yeast to give a genetically modified strain capable of producing large amounts of artemisinic acid 1. State two advantages of using whole cells to make compound 1 rather than using the three isolated enzymes.
(c) The cytochrome P450 and a cytochrome P450 reductase catalyse the oxidation reaction shown below:
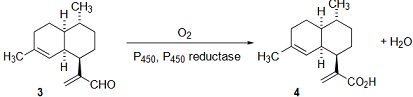
Draw a "curly-arrow" mechanism for this reaction showing how oxygen is activated by the metal center, and hence explain why the reductase is needed to complete the catalytic cycle.
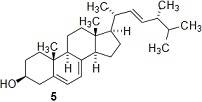
(d) Farnesyl pyrophosphate is also a precursor for ergosterol 5. Briefly explain why the existence of the pathway used to make ergosterol 5 might reduce the amount of artemisinic acid 1 made by the genetically modified yeast, and briefly explain how you might overcome this problem.
Question 2. (a) Using clear diagrams, briefly explain the difference in design between batch (BR) and continuous stirred tank (CSTR) reactors.
(b) The degree of substrate conversion, x, is defined as 1 - [S]0/[S] where [S]o is the initial substrate concentration and [S] is the concentration of substrate remaining. Another important parameter is the residence time of substrate in the reactor, τ. For a BR, one can write the following expression:
τ = [S]0∫dx/r
Assuming that the rate r can be approximated by the initial velocity vo, use the Michaelis-Menten equation to obtain the following expression:
x[S]0 + KM ln(1 - x) = Vmaxτ
(c) The inhibition of the biocatalyst can become very problematic when transformations must be carried out to high conversions at high substrate concentrations. In order to select the correct reactor, we have to identify the correct kinetic equation and then use it with the equation for residence time in the reactor that we propose to use for the conversion. Substrate inhibition is often observed, which can give a very slow initial rate. The kinetic mechanism for this type of inhibition can be shown as:
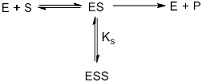
Based in this information, write down the modified form of the Michaelis-Menten equation for the initial velocity and obtain an expression for the product formed (Vmax.τ) if the transformation is carried out in a BR.
(d) Using the equation derived in question 2(c), briefly explain why you would choose to use a CSTR for the transformation when substrate inhibition is a significant problem.
Question 3. (a) Consider the following metabolic pathway, which is present in Escherichia coli. Arrows correspond to single or multiple enzyme-catalyzed steps. In answering this question, you do not have to worry about the role of glucose in any other metabolic pathway.
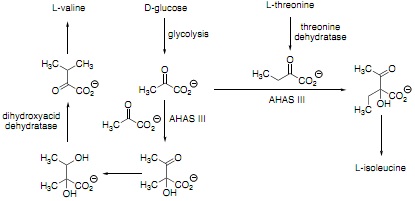
The enzyme AHAS III is inhibited by L-valine, which binds to a regulatory domain that is separate from the catalytic domain. Briefly explain how this feedback loop benefits the cell and propose a strategy to overcome this regulation.
(b) Briefly explain why "knocking out" the gene encoding PLP-dependent enzyme threonine dehydratase increases the amount of L-valine produced in the resulting "engineered" strain of Escherichia coli.
(c) Write a curly-arrow mechanism for the reaction catalyzed by dihydroxyacid dehydratase.
(d) In order to achieve the highest production of L-valine, the expression levels of genes encoding amino acid transporters were increased in the engineered strain of Escherichia coli. Give one example of how the expression level of a given gene can be increased, and briefly explain this finding given that the transporters are not directly involved in L-valine biosynthesis.
(e) The engineered strain of Escherichia coli produced 0.378 g of L-valine per gram of glucose provided to the bacterium. Calculate the efficiency of the overall transformation.
Question 4. (a) Starch and cellulose are both polymers of D-glucose, as shown below:
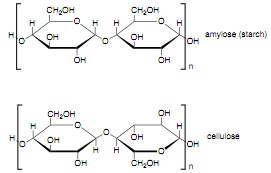
Identify the structural feature that is different in starch and cellulose and, using a clear diagram, explain how this feature leads to the very different physical properties of the two polymers.
(b) Starch is easily hydrolyzed into D-glucose by enzymes called hydrolases. Briefly explain why these enzymes cannot break down cellulose.
(c) Lytic polysaccharide monooxygenases (LMPOs) are Cu(II)-dependent enzymes that can breakdown cellulose into shorter chains by oxidatively breaking the glycolytic linkages, as shown below:
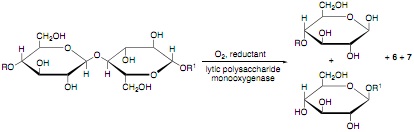
Draw structures for the reaction products 6 and 7.
(d) Propose two assays for measuring the kinetic properties of lytic polysaccharide monooxygenases and briefly explain why ascorbate must be included in your assay mixtures.