Reference no: EM133047029
Question 1. Answer ALL parts a) - d).
The amine1 is an anti-cancer drug that is made using the following reaction, which is catalysed by an imine reductase:
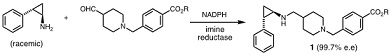
a) Write a curly-arrow mechanism for this reaction that clearly shows the involvement of NADPH.
b) State the maximum yield of this reaction and briefly justify your answer.
c) State two problems with using this reaction to obtain amine 1 in multi-gram amounts.
d) An engineered ketoreductase can be used to catalyse the following reaction:

Propose three advantages of using the ketoreductase and the imine reductase in an enzyme cascade to convert the alcohol 2 into the amine 1.
Question 2. Answer ALL parts a) - d).
Haloalkane dehalogenases are hydrolase enzymes that catalyse the conversion of alkyl halides to alcohols under mild conditions.
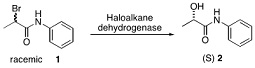
a) The enzyme active site contains crucial aspartate and histidine residues, requires a water molecule for reactivity and uses covalent catalysis. Describe the reaction mechanism of 1 to 2 by haloalkane dehalogenase using a curly arrow diagram.
b) Define the term enantiomeric excess (e.e.)
c) Using diagrams to aid your answer as necessary, explain why at greater conversions of 1 the enantiomeric excess of (S)-2 will be lower.
d) Addition of the insoluble phosphonium bromide 3 to the reaction transforms the process such that (S)-2 can be isolated with 98% e.e. and 78% yield.

Explain, with the aid of appropriate diagrams how such a dynamic kinetic resolution is able to circumvent the limitations of a standard kinetic resolution and achieve both high yield and high e.e.You should include a curly arrow mechanism in your answer that explains the role of 3 in this specific example.
Question 3. Answer ALL parts a) - e).
Levetiracetam, an anticonvulsant drug may be prepared using a part-biocatalytic synthesis as outlined below.
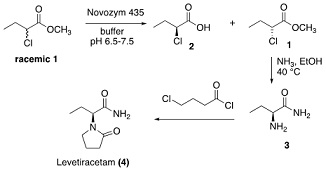
a) The initial step is a kinetic resolution. State the stereochemical selectivity of the enzyme (Novozym 435) active site for this substrate, justifying your answer briefly.
b) It was found to be unnecessary to separate the products 1 and 2 prior to chemical reaction with ammonia to generate 3 as single enantiomer. Propose a reason for this and state how unwanted 2 is hence removed from 3 as a result.
A researcher has proposed that this reaction could be converted into a dynamic kinetic resolution through carrying out this process with an alkaline stable serine hydrolase at pH 9.0 or above.
c) Provide a rationalisation of how this approach would work and state why the presence of the chlorine atom may promote this process. You should include suitable diagrams to illustrate any chemical processes taking place (the catalytic mechanism of the enzyme is not required).
d) In practice the DKR proposed in step c) proved to be unsuccessful even though the enzyme itself was stable to the conditions tested (at pH 9.0, 10.0 and 11.0). Suggest a reason why this process was a failure.
A company wishes to develop a biocatalytic route using dehydrogenase enzymes to the following compounds, starting from triketone 5.
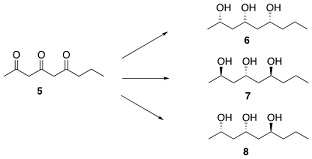
e) Provide specific enzymes and cofactors to achieve the three desired outcomes, justifying your answer with reference to diagrams and showing that one of the three compounds may only require a single enzyme with a single cofactor recycling system.
Hint: you may use lecture notes and refer to the required stereoselectivity at each new stereocentre.