Reference no: EM131034747
1. Using your knowledge of particles, explain why the process of evaporation has a cooling effect on the skin.
2. How would you describe the difference between a chemical change and a physical change? In your answer provide an example of each.
3. Tap water contains many dissolved minerals and gases. With this in mind, would you consider water to be a heterogeneous mixture or a homogeneous mixture? Explain your choice.
4. Briefly describe what is meant by the term "emulsion".
5. Students in Foundation Science have discovered two new elements and have given them the symbols, Fs-1 and Fs-2. (These new elements are fictitious and of course not found on the Periodic Table). The average atomic mass the elements are Fs-1 = 114 g/mol and Fs-2 =125 g/mol. Both elements are neutral in charge and have 65 electrons present. With this information, please answer the following.
a) What is the atomic number of the element Fs-1?
b) What is the atomic number of the element Fs-2?
c) Is it possible that Fs-1 and Fs-2 are isotopes? Justify your answer.
6. Refer to a Periodic Table to answer the following questions.
a) Where are the noble gases located?
b) Are there any metals in period 1?
c) How many electrons are present in an atom of iodine (I)?
d) What is the average atomic mass for Magnesium (Mg)?
e) How many protons are there in an atom of Phosphorus (P)?
f) How many electrons are present in an atom of iron, (Fe)?
g) Which element has an atomic number of 10?
h) Which element does not have any neutrons?
i) Give the symbol for the alkali metal in Period 2.
j) Is the element in Period 3, Group 2 a metal or a nonmetal?
k) What is the symbol for the halogen in Period 3?
l) Give the name of a non-metal in Group 1.
7. What is the difference between ionic bonding and covalent bonding?
8. Consider the molecule represented by the following chemical structure:
By carefully considering the representation of atoms in the diagram, write the molecular formula for this molecule.
9. If a solution NaOH (sodium hydroxide) has a concentration of 0.050 mol/L, calculate the following.
a) The concentration of this solution in grams per litre (g/L).
b) The concentration of this solution in %w/v.
10. What volume of a 0.25 M solution is required to prepare 3.5 L of a 0.10 M solution?
(Note: M = mole per Litre) (Hint: determine the dilution factor required).
11. The metabolism of glucose in the presence of oxygen is shown by the following reaction:
C6H12O6(aq) = 6O2(g) ⇔ 6CO2(g) + 6H2O(l)
a) Show that this reaction is balanced by tallying the number of each type of atom present on either side of the equation.
b) If 4 mole of glucose reacts with excess oxygen, O2, how many mole of carbon dioxide, CO2, would be produced?
12. Consider the following diagram for a reaction pathway without a catalyst.
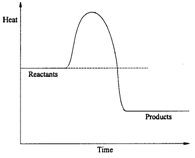
a) Does this diagram represent an endothermic or an exothermic reaction? Explain your answer.
b) What is meant by the term 'activation energy'?
c) If a catalyst were introduced into the reaction, how would the illustrated reaction pathway change? d) If a catalyst is used, will it be consumed in the reaction?
e) If a catalyst is used, will it affect the yield of the reaction?
f) If a catalyst is used, will it affect the rate of the reaction?
13. The pH scale of acidity is used to describe acids and bases. Complete the following table by matching each of the three given pH values with the solution that it would describe.
|
pH value
|
Matches with(write letter for correct solution description)
|
Solution description
|
|
1.1
|
|
a) Neutral solution
|
|
7.0
|
|
b) Slightly basic solution
|
|
3.5
|
|
c) Slightly acidic solution
|
|
8.0
|
|
d) Very basic solution
|
|
6.3
|
|
e) Very acidic solution
|
|
11.3
|
|
f) Moderately acidic solution
|
14. Answer the following questions regarding buffers. Remember that a buffer contains a weak acid and its conjugate base.
a) Name the buffer present in human blood that is essential in maintaining a pH between 7.35 and 7.45.
b) Briefly describe how the buffer responds to an increase in the amount of acid in the blood, in order to maintain blood pH within the range of 7.35 and 7.45.Include relevant chemical reactions in your answer.
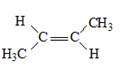
15. Complete the table below by Identifying the functional group, the number of carbons present in the chain, the parent alkane and the IUPAC ending that would be used to name the molecule. The first molecule has been completed for you as an example.
|
Molecular representation
|
Functional group
|
Number of carbon atoms present in longest continuous chain
|
Alkane that the molecule name will be based on
|
IUPAC ending
|
|
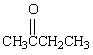
|
ketone
|
4
|
butane
|
-one
|
|
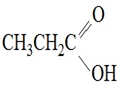
|
|
|
|
|
|
|
|
|
|
|
|
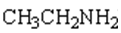
|
|
|
|
|