Reference no: EM131044510
1. Explain how Quantum Mechanics changes the deterministic view of Nature that existed in Newtonian physics.
2. Consider the electromagnetic spectrum:
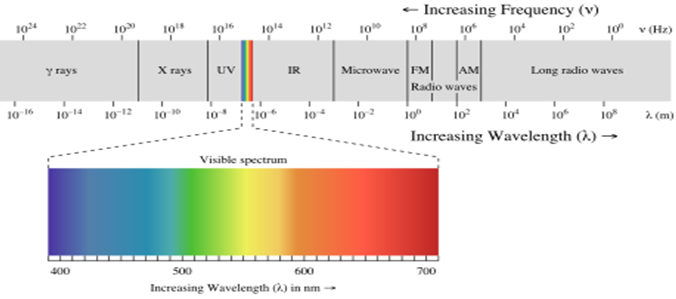
Arrange photons of the following types of radiation according to the energy of the individual photons. From highest to lowest energy: infrared, blue light, yellow light, ultraviolet, γ rays, AM radio waves, X-rays, microwaves.
3. Suppose that you were doing the Young double-slit experiment, and that you could decrease the intensity of the light greatly (so much that individual photons were impacting the screen). What would you see and why?
4. Suppose that a green light beam has a controllable intensity (i.e., it can be made more of less bright). As you increase the intensity (i.e., the number of photons in the beam), do the energies of the photons of green light increase, decrease, or stay the same? Explain
5. Suppose that a proton and an electron have both the same speed of 1X106 m/s. What are their respective deBroglie wavelengths?
6. What is the deBroglie wavelength of a 2 kg bowling ball moving with a speed of 4.5 m/s?
Discuss whether you would expect to be able to observe diffraction phenomena for the bowling ball.
7. What is the energy of a photon of wavelength 400 nanometers?
And that of a photon of wavelength 700 nanometers? (These correspond to violet and red light, respectively).
8. An electron has a speed v. Another electron has speed (1/3)v. What is the ratio of the deBroglie wavelengths of these two electrons?
9. What would happen with the quantization of energy if we were able to change, at will, Planck's constant and make it 0?
10. If Planck's constant, h, were smaller than it is, how would the uncertainty principle be affected? What if h were zero?
11. An electron has an uncertainty in its velocity of 0.1 m/s. The mass of the electron is 9.1x10-31 kg. Calculate the uncertainty in its momentum. Using this result, calculate what the minimum uncertainty in the position of this electron must be in order to obey the Heisenberg Uncertainty principle.
12. Now consider a proton with an uncertainty in its velocity of 0.1 m/s. The mass of the proton is 1.7x10-27 kg. Calculate the uncertainty in its momentum. Using this result, calculate what the minimum uncertainty in the position of this proton must be in order to obey the Heisenberg Uncertainty principle.
Compare the results of problems 11 and 12: can you arrive at some general conclusion?
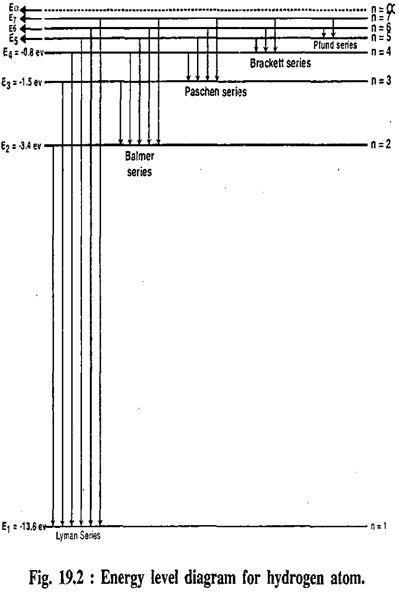
13. Among the 10 quantum jumps between the five energy levels of hydrogen shown in Figure 13.21, which one produces the photon with the lowest frequency?
14. Among the 10 quantum jumps shown in the figure, which produces a photon with the highest frequency?
15. The energy difference
E2-E1 is 16x10-19 J. Determine the frequency of the photon emitted in the transition from state 2 to state 1. Determine the wavelength of the photon. Determine to which region of the electromagnetic radiation spectrum does this photon belong to.
|
Determining the exercise of the options
: At the date of grant, the stock was selling for $19 per share. Laura exercised the options this year when the market price was $28 per share. How much income must Laura recognize from the exercise of the options?
|
|
How you believe this training would improve the workplace
: Describe the importance and characteristics of interpersonal relationships in the workplace.
|
|
Dollar cost to dow chemical of buying
: (a) How many U.S. dollars will Dow Chemical receive from the sale of U50 million? (b) What is the U.S. dollar cost to Dow Chemical of buying U1 billion?
|
|
Theme and description by answering these
: Wite two brand blogs. The first one should be about (chanel bags). The second one you can choose any brand you want. please make sure you cover all the asked part below on each blog.
|
|
Which produces a photon with the highest frequency
: An electron has an uncertainty in its velocity of 0.1 m/s. The mass of the electron is 9.1x10-31 kg. Calculate the uncertainty in its momentum. Using this result, calculate what the minimum uncertainty in the position of this electron must be in o..
|
|
Monthly payment amount on the loan
: You are planning to buy your first house. The cost of the house is $200,000, of which you will pay 20% as a down payment and finance the remainder. The mortgage rate on the 30-year loan with monthly payments is 6% compounded monthly. What is the m..
|
|
Direct quote for the real in bangkok
: 1. Suppose the quote on pounds is $1.9721-35/£. If you converted $10,000 to pounds and then back to dollars, how many dollars would you end up with? 2. Suppose the Brazilian Real is quoted at $0.9455-9510, and the Thai baht is quoted at $25.2513-..
|
|
What is the company net income for 2015
: What is the company's net income for 2015? (Do not round intermediate calculations. A negative answer should be indicated by a minus sign.)
|
|
The use of social media in public relation
: Think of an organization that you admire. Reflect on its social media presence (Facebook, Twitter, YouTube... etc.). Evaluate the organization's use of social media, specifically in terms of its themes and messaging and the effectiveness of its co..
|