Reference no: EM131129672
Part A:-
Matching terms. Choose the best answer from the following list below and assign a number. Not all terms will be used.
1. Ethanol
2. Kinase
3. Acetaldehyde
4. Phosphatase
5. Carbon dioxide
6. 2,3 bisphosphoglycerate
7. Mutase
8. Isomerase
9. Pyruvate
10. Oxygen
A. _____ In the glycogen catabolism pathway shown on page 6, this class of enzyme catalyzes reaction 2.
B. _____ In yeast fermentation, it is the final electron acceptor.
C. _____ This molecule allosterically increases hemoglobin's affinity for oxygen.
Part B:-
Fill in the blank. Write in the FULL name of the structure.
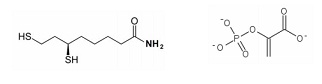
1.__________________2._________________________
Part C:-
Multiple Choice. Circle the best answer.
1. Suppose the E3 enzyme of pyruvate dehydrogenase complex could no longer reduce NAD+ to NADH. Which of the following statements is FALSE about the complex?
a. E3 would contain bound FADH2.
b. The Lipoamide arm would be reduced.
c. The hydroxyethyl intermediate would be bound to TPP.
d. An acetyl group would be bound to the lipoamide arm.
e. None of the above, i.e. all are true!
2. If the peptide below was cleaved with chymotrypsin, the products would be:
Ser-Met-Glu-Tyr-Val-Asn-Gly
a. One peptide 5 residues long and one peptide 2 residues long.
b. One peptide 4 residues long, one peptide 2 residues long, and one free amino acid.
c. One peptide 6 residues long and one free amino acid.
d. One peptide 3 residues long and two peptides 2 residues long.
e. One peptide 4 residues long and one peptide 3 residues long.
3. Which of the following is ONLY a diastereomer of fructose (and nothing else!)?
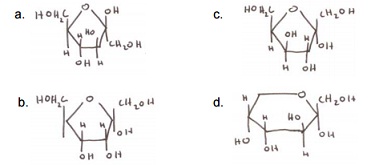
Questions 4 and 5 refer to glycogen catabolism:-
4. Which of the monosaccharide phosphate molecules listed can mutarotate?
a. Glucose-1-phosphate.
b. Glucose-6-phosphate.
c. All of the above.
d. None of the above.
5. What is the ATP yield for a cell catabolizing one residue of glucose from glycogen under hypoxic (low oxygen) conditions?
a. Three.
b. One.
c. Two.
d. Four.
e. None of the above.
6. Consider a mutation in hemoglobin where the proximal histidine (the histidine is normally bound to the heme iron) in each subunit is converted to alanine. Compared to the O2 binding curve of wild type hemoglobin:
a. The mutant would shift the curve to the right.
b. The mutant would switch the curve from sigmoidal to hyperbolic.
c. The mutant would shift the curve to the left.
d. The mutant would not change the curve.
7. Which of the following does NOT allosterically inhibit an enzyme in glycolysis?
a. ATP.
b. NADH.
c. Citrate.
d. Glucose-6-phosphate.
e. None of the above.
Part D:-
Short Answer Questions:
1. Given the following reaction:
3-phosphoglycerate (3PG) + ATP ↔ 1,3 Bisphosphoglycerate (1,3BPG) + ADP
?Go' = 18.5kJ/mole
a. What is the enzyme that catalyzes this reaction?
b. Calculate the lowest ratio of 3PG/1,3BPG that would allow the reaction to be spontaneous at physiological conditions. Assume the ratio of [ATP]/[ADP] has a value of 8. Do you think this reaction could occur within a cell? Why?
2. Draw out the overall reaction catalyzed by glyceraldehyde-3-phosphate dehydrogenase. Make sure to include all names and structures.
3. When fatty acids are used as an energy source, they are initially broken down into acetyl-CoA and NADH in the mitochondrial matrix by a process known as β-oxidation. How would a high level of β-oxidation of fatty acids affect pyruvate dehydrogenase complex? Provide a DETAILED rationale for your answer!
4.Carbon monoxide (CO) binds to heme iron in hemoglobin (Hb) in a manner very similar to O2; however, it binds with a much higher affinity. On the graph below, draw the O2 binding curve for Hb in the presence of CO (you may assume ~1 CO per hemoglobin molecule). The O2 binding curve for Hb in the absence of CO has been provided as a reference. Provide a brief explanation for the curve you drew.
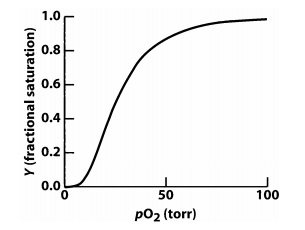
5. A mutation in chymotrypsin exists that places an aspartate's side chain beside oxyanion hole. Base on your knowledge in class, provide a detailed explanation on how this mutation would affect chymotrypsin's catalytic mechanism.
______________________________________________________________________
Glycogen breakdown:-
Glycogen (a polymer of glucose) is broken down as shown below:-
Reaction 1: Glycogen(n) + Pi → Glycogen(n-1) + Glucose-1-phosphate
Reaction 2: Glucose-1-phosphate → Glucose-6-phosphate
Where Pi = Inorganic phosphate
______________________________________________________________________
Equations & Constants:
K = oC + 273
ΔG = ΔH - TΔS
F: 96,480 J/V mol
ΔGo' = -nFΔEo
'
R: 8.315 J/mol K
ΔG' = ΔG'o + RT ln ([C][D]/[A][B])
1 kDa: 1000 g/mole
Vo = Vmax([S]/([S] + Km))
Where A & B are reactants and C & D are products
pH = pKa + log([A-]/[HA])
1/Vo = 1/Vmax + (Km/Vmax)(1/[S])