Reference no: EM131260710
Electron Shielding and Effective Nuclear Charge
Critical Thinking Exercise
This exercise uses an Excel spreadsheet template to predict the strength of the attractive force acting on an atom's valence electrons. The file 'effective nuclear charge sim - Part 1.xlics' is available on CANVAS within the "Exercises" module. Download and open this file in Excel to make the observations and answer the questions below.
I. Collect and plot data:
The number of protons and electrons in an atom can be set using the widgets on the left. The extent to which the outermost electron (last one added) is shielded from the positive charge of the nucleus is calculated by the spreadsheet and subtracted from the actual nuclear charge (Z, atomic number) to get the effective nuclear charge, Zeffective, that attracts the outermost valence electron.
Use the simulation to determine the Zeffective for neutral atoms of the elements sodium through calcium. Create a graph of both Z and Zeffective on the y-axis plotted vs. the number of protons (Z = 11 through 20) on the x-axis. Use the Scatter Plot graphing option for this. Your graph will have two sets of data on the same graph, one of wriit.n viii De a straight diagonal line. Be sure to label your graph appropriately and print a copy to turn in with this exercise.
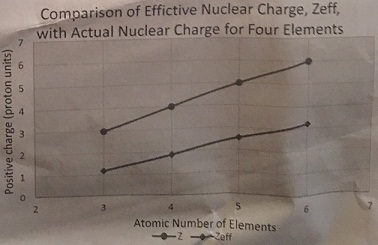
An example table and graph is shown below for a series of 4 elements, lithium through carbon:
|
Atomic No.
|
Actual charge Z
|
Effective charge Zeff
|
|
3
|
3
|
1.2
|
|
4
|
4
|
1.88
|
|
5
|
5
|
2.55
|
|
6
|
6
|
3.23
|
II. Use your graph and the simulation to answer the following quesktI4nst
1) For the elements across the 3rd row of the Periodic Table (Na through Ar), the actual nuclear charge, Z, increases by 7 proton units.
a) By how much (in proton units) did the effective nuclear charge, Zeff increase) (Be sure the number of electrons is the same as the number of protons for each element so that you are comparing neutral atoms!)
b) Which element in the row exerts the greatest attraction on its valence electron(s), and which element exerts the least attraction on its valence electron(s)?
2) How does the Zeff for potassium compare with the Zeff for argon? Explain why the trend in Zeff suddenly reverses when another proton and electron are added to Ar to create a K atom.
3) Create a potassium cation, K+, by removing one electron from the potassium atom to leave it with a positive charge.
a) How does the Zeff for K+ compare with the Zeff for a neutral potassium atom? Try to explain what you observed.
b) How does the Zeff for K+ compare with the Zeff for a neutral atom of argon? Try to explain what you observed.
|
Trend in american medicine-an entire
: There is a major new trend in American medicine-an entire new professional physician group known as "hospitalists." Currently the American public has a very poor understanding of what a hospitalist is and what role he or she performs in patient ca..
|
|
Identify any gradually varied flow profiles
: Determine what changes take place due to the presence of the transition. In your analysis include all relevant rapidly varied flow calculations, identify any gradually varied flow profiles, and sketch the water surface and energy grade line.
|
|
Create and initialize an array called testmarks
: Write a program that will prompt the user to enter his/her name and a number. Use a while loop that will display the user name times the entered number - Prompt the user to enter a mark and store an inputted value on the variable mark
|
|
Write a research about origin of flowering plants
: Write a research about Origin of Flowering Plants for Botany majer. The format of citation is American Journal of botany, and all resources must be primary resources.
|
|
Which element in the row exerts the greatest attraction
: Which element in the row exerts the greatest attraction on its valence electron(s), and which element exerts the least attraction on its valence electron(s)?
|
|
Formulate watsons production mix decision
: Formulate Watson's production-mix decision as a linear programming problem, and solve. How many tables and bookcases should be produced each week? What will the maximum profit be?
|
|
Find the nature of the water surface and energy grade line
: The discharge is 33 m3 /s. At the entrance to the channel, the depth is 0.68 m, and at the downstream end a free-outfall condition exists. Perform a profile synthesis to determine the nature of the water surface and energy grade line.
|
|
Write out the objective and constraints
: A craftsman named William Barnes builds two kinds of birdhouses, one for wrens and a second for bluebirds. - Write out the objective and constraints.
|
|
Implementation of community health information network
: 1a. From the e-Activity, determine a key trend that supports the implementation of either a community health information network (CHIN) or regional health information organizations (RHINO) in today%u2019s health care organizations. Justify your re..
|