Reference no: EM13926364
1 How fast is an electron moving if it has a wavelength equal to the distance it travels in one second?
(A) √h/m
(B) √m/h
(C) √h/p
(D) √h/2KE
2. According to Bohr's theory the radius of electron in an orbit described by principal quantum number n and atomic number Z is proportional to
(A) Z2n2
(B) Z2/n2
(C)Z2/n
(D) n2/Z
3. An excited H-atom emits electromagnetic radiations during de-excitation as shown in the given figure. Which of the following is correct relationship among the following wavelengths?
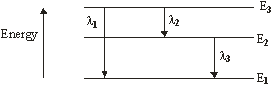
(A) λ1 = λ2+λ3
(B) λ1 = λ2*λ3
(C)1/ λ1 = λ2+λ3/λ2λ3
(D) 1/λ1 = λ2-λ3/λ2λ3
4. Which is not possible value of energy in case of hydrogen atom.
(A) -13.6 eV
(B) -3.4 eV
(C) -1.51 eV
(D) -5.2 eV
5. What is energy of electron in 2nd -shell for He+ ion?
(A) - 13.6 eV
(B) - 6.8 eV
(C) - 3.4 eV
(D) - 3.51 eV
6. The two electrons in the K-shell(1st -shell) will differ in
(A) principal quantum number
(B) Azimuthal quantum number
(C) magnetic quantum number
(D) spin quantum number
7. Which combination of quantum number n, l,m,s for the electrons in an atom does not provide a permissible solution of the wave equation ?
(A) 3, 2,-2, ½
(B) 3, 3, 1, - ½
(C) 3, 2, 1, ½
(D) 3,1,1, - ½
8. The wave number of first line of the balmer series of hydrogen is 15200 cm-1. The wave number of the first balmer line of Li2+ ion is
(A) 15200 cm-1
(B) 60800 cm-1
(C) 76000 cm-1
(D) 136800 cm-1
9. The kinetic energy of a proton is increased nine times the wavelength of the debroglie wave associated with, it would become
(A) 3 times
(B) 9 times
(C) times
(D) times
10. The energy of the second orbit of hydrogen is equal to the energy of
(A) fourth orbit of He+
(B) fourth orbit of Li2+
(C) second orbit of He+
(D) second orbit of Li2+
11 If the speed of electron in the in the Bohr's first orbit of hydrogen atom be x, then the speed of the electron in the third Bohr orbit of hydrogen atom is
(A) x/9
(B) x/3
(C) 3x,
(D) 9x
12. An electron has spin quantum number and a magnetic quantum number-3, It can be represented in
(A) s-orbital
(B) p-orbital
(C) d-orbital
(D) f-orbital
13. Which of the following electron transition in a H-atom will require the largest amount of energy?
(A) from n = 1 to n = 2
(B) from n = 2 to n = 3
(C) from n = ∞ to n = 1
(D) from n = 3 to n = 5
14. The ratio of difference between 1st and 2nd Bohr's orbits energy to that between 2nd and 3rd orbits energy is: (for any single electron species)
(A) 1/2
(B) 1/3
(C) 27/5
(D) 5/27
15. If the Planck's constant h = 6.6*10-34 Js, the de-Broglie wavelength of a particle having momentum 3.3*10-24 Kg ms-1 will be :
(A) 0.02
(B) 0.5
(C) 2
(D) 500
16. Transition of an electron from n = 4 to n = 2 level results (for an H - atom)
(A) IR spectrum
(B) UV spectrum
(C) Visible spectrum
(D) X - ray spectrum
17. The ratio of energy of a photon of KÅ wavelength radiation to that of 4KÅ radiation is
(A) 4/2
(B) 1/4
(C) (4)2
(D) (2)2
18. Which electronic level would not be possible?
(A) 3s
(B) 1p
(C) 2s
(D) 2p
19. Possible value of angular momentum of orbit would be
(A) h/2Π
(B) 2h/2Π
(C) 3h/2Π
(D) All of these
20. When the azimuthal quantum number (l) is 3, m can have
(A) 1 value
(B) 4 values
(C) 5 values
(D) 7 values
|
Should the manager invest in the new equipment
: An employee has proposed adding new equipment that would speed up the loading rate to 4.2856 trucks per day. The equipment would cost $100 per day for each dock. Should the manager invest in the new equipment?
|
|
Probability of an event not occurring
: The Complement rule states that the probability of an event not occurring is
|
|
What number of clerks would be optimal in terms of minimizin
: If clerks represent a cost of $20 per hour and mechanics a cost of $30 per hour, what number of clerks would be optimal in terms of minimizing total cost?
|
|
Normal distribution and probability theory
: Rudimentary probability theory is the primary focus of this section. Since many everyday situations are based on probabilistic reasoning and occurrences, it is important to have an understanding of probability theory and its connection to statisti..
|
|
Which electronic level would not be possible?
: An excited H-atom emits electromagnetic radiations during de-excitation as shown in the given figure. Which of the following is correct relationship among the following wavelengths?
|
|
Correlation-predication-confidence and errors
: This section examines correlation, estimating confidence, and margin of errors. Correlation is an important concept to understand, since often what is found in many areas of research is not cause, but a relationship that exists among variables.
|
|
Identify the most effective organizational structures
: Identify the most appropriate and effective organizational structures for Riordan Manufacturing that will help them accomplish their planned changes.
|
|
The three principles that accrual accounting
: Which is not one of the three principles that accrual accounting is based on?
|
|
Produce an analysis class diagram
: Produce an analysis class diagram focusing on the problem domain. At this point it is not required to be fully attributed. However it is important that you attempt to model as many of the entities as possible, and to ensure the correct multiplicit..
|