Reference no: EM132373244
Assignment - Biochemistry Questions
FOR Q1 - 9 Figure 1 is a Michaelis-Menten plot for two different enzymes (I and II.)
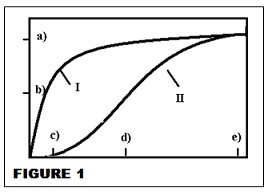
Q1. The x-axis is:
a) V b) [O2] c) P50 d) KM e) [S].
Q2. Which has the larger Vmax?
a) I b) II c) they are both about the same.
Q3. Which forms the transition state (ES complex) more easily at lower [reactant]?
a) I b) II c) they are both about the same.
Q4. Which has the lower [Vmax]/2?
a) I b) II c) they are both about the same d) this is a stupid question: speed is not an issue.
Q5. Which has the higher KM?
a) I b) II c) they are both about the same d) this is a stupid question: KM is about myoglobin.
Q6. Which of these is NOT true?
a) Enzyme I has a lower KM.
b) Enzyme II has a lower kcat.
c) Enzyme II has a lower specificity constant.
d) Enzyme II is allosterically affected.
e) Enzyme II is more likely to be a control point in a pathway.
Q7. The Lineweaver-Burk plot for enzyme I is y = 5x + 1. Which ONE of these could be the L-B plot for enzyme II?
a) 5y = x + 1 b) y = 0.2x + 1 c) y = 0.2x + 5 d) y = 25x + 25 e) y = 25x + 1
Q8. The Lineweaver-Burk plot for enzyme I is y = 5x + 1. If [active site] = 1 for both enzymes, then the kcat for enzyme I is ___ of enzyme 2.
a) greater than that b) less than that c) about the same as that.
Q9. The Lineweaver-Burk plot for enzyme I is y = 5x + 1. If [active site] = 1 for both enzymes, then the specificity constant for enzyme I is ___ of enzyme 2.
a) greater than that b) less than that c) about the same as that.
FOR Q10 - 13 Figure 1 is a Michaelis-Menten plot for glucosidase (a hydrolyase enzyme) under condition I or condition II.
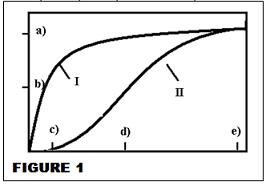
Q10. Which condition suggests the presence of a competitive inhibitor?
a) I b) II c) both d) neither.
Q11. Which condition suggests the presence of an allosteric inhibitor?
a) I b) II c) both d) neither.
Q12. Which condition suggests the presence of a non-competitive inhibitor?
a) I b) II c) both d) neither.
Q13. The slope of an L-B curve for this enzyme with cellulose as a reactant is less than the slope of an L-B curve for this enzyme with lactose as a reactant. Which line shows the M-M plot of this enzyme with cellulose?
a) I b) II.
Q14. Consider the information in Q13. Which of the following probably not true according to this infor and the graphs?
a) This enzyme is quite stereo-specific.
b) The M-M plots for glycogen and sucrose as reactants would be considerably lower and broader for this enzyme (maybe even flat!)
c) This enzyme has a large active site.
d) This enzyme's activity involves very few interactions with the reactant.
e) Lactose is the natural substrate for this enzyme.
Q15. You learn that this enzyme has an activity plot shown in Figure 2. Which ONE of these pairs of parts of a protein would be responsible for such a curve?
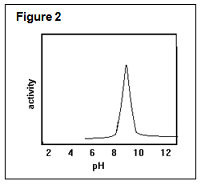
a) his-lys b) lys-arg c) cys-his d) his-α-amino e) α-carboxyl-asp
Q16. Which ONE of these explanations would be accurate for this curve?
a) the first group in the pair would be acting as an acid; the second as a base.
b) the first group in the pair would be acting as a base; the second as an acid.
c) the first group in the pair would be acting as a cation; the second as an anion.
d) the first group in the pair would be acting as an anion; the second as a cation.
e) the first group in the pair would be acting as an electrophile; the second as a nucleophile.
Q17. According to the correct answer for Q86 above for a glucosidase, which of the following would be the correct transition state for by the first group acting?
a) a carbocation b) a carbanion c) a pentavalent carbon d) an oxycation e) a metal hydride.
Q18. According to the correct answer for Q87 above for a glucosidase, the other reactant for this enzyme would be the _____ reaction with the ___.
a) oxygen of water; carbocation b) oxygen of water; carbanion c) hydrogen of water; carbocation d) hydrogen of water; carbanion e) MTOON
Q19. The products of a glucosidase-catalyzed reaction could be:
Q20. If glucosidase is mixed with a large concentration of glucose.