Reference no: EM132618387
Question 1: Glycerine at 10 °C flows over a wide. 1 m long, heated flat plate at 2 m s-1. For a plate surface temperature of 30 °C, determine:
(a) The average convective heat transfer coefficient
(b) The heat transfer rate per unit width in kW m-1
(c) The heat transfer rate per unit width in kW m-1 if the fluid is ammonia, and discuss the factors that contribute to the difference of value determined far this fluid as compared to the value calculated for glycerine in (b), above (max 150 words),
(d) What would the governing heat transfer mechanism be if the velocity of the glycerine flow were decreased by a factor 10?
Data:
Glycerine properties at different temperatures
|
T (°C)
|
Density (kg m-3)
|
Kinematic viscosity (m2
s-1)
|
Thermal conductivity (W
m-1 K-1)
|
Prandtl number (-)
|
β (K-1)
|
|
o
|
1276.03
|
1100831
|
0.282
|
84700
|
0.50 x 10-3
|
|
10
|
1270,11
|
0.00300
|
0_284
|
31000
|
|
20
|
1264.02
|
0.00118
|
0.286
|
12500
|
|
30
|
1258.09
|
0.00050
|
0.286
|
5380
|
Ammonia properties at different temperatures
|
T (°C)
|
|
Density
(kg m-3)
|
|
Kinematic viscosity (m2
s-1)
0.373 x 10-6
|
Thermal
conductivity (W m-1 K-1
0.540
|
|
.
Prandtl number (-)
2.05
|
|
|
β (K-1)
2.45 x
10-3
|
|
0
|
|
640,10
|
|
|
10
|
|
626.16
|
|
0.368 x 10-6
|
0.531
|
.
|
2.04
|
|
|
20
|
|
611_75
|
|
0.359 x 10-6
|
0.521
|
|
2.02
|
|
|
30
|
|
596.37
|
|
0.349 x 10-6
|
0.507
|
|
2.01
|
|
Heat transfer correlations for a flat plate (all fluid properties evaluated at the film temperature; Rey is the Reynolds number at the end of the plate)
|
Fluid flow conditions
|
Correlation
|
|
Natural convection (turbulent regime)
|
Nu = 0.02 1Ra2/5
|
|
Forced convection (laminar regime}
|
Nu = 0.66 4Re1/2Pr1/3
|
|
Forced convection (turbulent regime)
|
Nu = Pr1/3(0.036 ReL0.8 - 836)
|
Question 2. The laminar now of a stratified oil and gas Row subject to gravity can be studied by the use of a simplified Cartesian model between two parallel flat plates inchned at an angle a with respect to the horizontal (Figure IL). Assume that the oil phase (density pi and viscosity pi) does not mix with the gas phase (density pz << pi and viscosity p2 ‹A pi), Using the continuity equation and the NaviermStolces equations, determine at steady state:
a) The velocity profile of the oil phase as a function of its thickness h1 clearly Stating your assumptions
b) The velocity profile of the gas phase as a function of its thickness h2, dearly stating your assumptions
c) The thickness h1 if the Oil volumetric flow rate per unit width q1 (m3 s-1 m-1l) is known, clearly stating your assumptions. The volumetric flow rates (m3 s-1) = q1 x width, can be computed as
Q1 = ∫∫A u1 dA1
where A1 is the cross-sectional area to the oil flow velocity (u1).
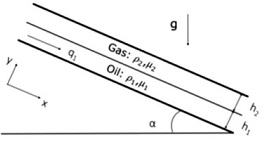
Figure 1 Oil and gas flow sketch on an x-y rectangular coordinate system
Data: For a 2D steady state, incompressibic fluid flow with a velouty field U = (u, v):
Continuity equation:
∂u/∂x + ∂v/∂y = 0
Question 3. A liquid hydrocarbon stream containing undesired sulphur-based compounds - abbreviated as HC-S- enters the top of a packed bed at a concentration CHC-5,0 = 5 mol m-3 and a velocity UL = 3 x 10-3 m s-1. As the liquid flows down the column, HC-S is adsorbed onto the surface of a solid zeolite, whose saturation concentration is CHC-5,sat = 2 moles m-3.
Data:
• Bed length = 5 m
• Bed diameter = 015 m
• The HC-S diffusivity constant to the zeolite, DHC-S = 10-8 m2 s-1
• The mass transfer coefficient of HC-5 from the liquid to the solid zeolite surface, kav = 0.002 s-1
(a) Calculate the value for the mass Peclet number, Pe, of HC-S in the liquid phase.
(b) Based on your answer to (a) above, what is the governing mass transfer mechanism within the packed bed?
(C) The unidirectional mass conservation equation for a chemical species i flowing down the packed bed described in the problem above is given by the expression
∂Ci/∂t + u∂Ci/∂z = Di ∂2Ci/∂z2 + Ri
Giving reasons, simplify the above equation to model the concentration of HC-S in the liquid as it flows down the bed under steady state conditions.
(d) Specify the boundary condition required to solve the simplifieri mass conservation equation derived in (C), above.
(e) Integrate the simplified equation derived in (c) subjected to the boundary condition from (d) to show that the HC-S concentration profile, CHC-S varies exponentially down the length of the bed.
(f) What length of zeolite bed would be needed to achieve a 50% removal of the HC-S from the liquid stream?