Reference no: EM131111308
ORGANIC CHEMISTRY
1. Which equation represents the combustion of methane with the products collected at 120 °C?
A CH4(l) + 2O2(g) → CO2(g) + 2H2O(l)
B CH4(g) + 2O2(l) → CO2(s) + 2H2O(l)
C CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
D CH4(l) + 2O2(l) → CO2(l) + 2H2O(s)
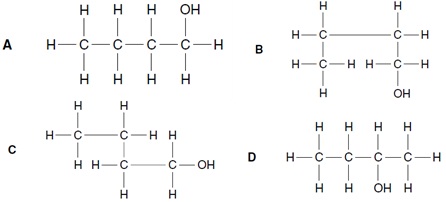
2. The structure of butan-1-ol is shown.
Which structure is an isomer of that shown above?
3. The diagram shows the fractional distillation of petroleum.
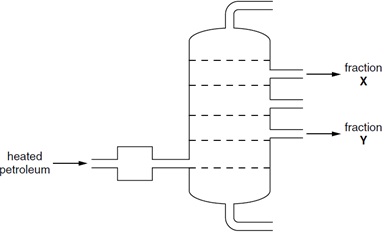
Which statements about fractions X and Y are correct?
|
X burns more easily than Y |
X has a higher boiling point than Y |
| A |
yes |
yes |
| B |
yes |
no |
| C |
no |
yes |
| D |
no |
no |
4. Which set contains all the possible combustion products of methane, CH4?
A carbon, carbon dioxide, carbon monoxide and water
B carbon, carbon monoxide and hydrogen
C carbon dioxide, carbon monoxide, hydrogen and water
D carbon monoxide and water
5. The structures of two compounds are shown.
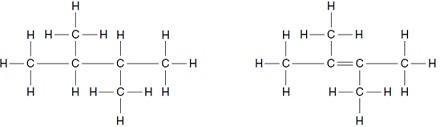
Which statement about these two compounds is correct?
A They are both hydrocarbons.
B They are both saturated compounds.
C They are in the same homologous series.
D They are isomers of each other.
6. Equal masses of coconut oil, butter, margarine and palm oil are separately dissolved in an organic solvent. A few drops of aqueous bromine are added to each solution and the mixtures are shaken.
The table shows the results. Which sample contains the most unsaturation?
|
sample |
colour of mixture |
| A |
butter |
orange |
| B |
coconut oil |
dark orange |
| C |
margarine |
yellow |
| D |
palm oil |
colourless |
7. When an animal fat is boiled with aqueous sodium hydroxide, a soap and glycerol are formed.
This reaction is an example of
A esterification. B fermentation.
C hydrolysis. D polymerisation.
8. The structure of the monomer of Perspex is shown.
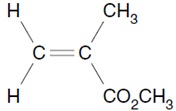
Which description of Perspex is correct?
|
type of polymer |
polymer formed by |
| A |
carbohydrate |
condensation polymerisation |
| B |
ester |
addition polymerisation |
| C |
hydrocarbon |
addition polymerisation |
| D |
polyester |
condensation polymerisation |
9. The apparatus shown was set up to prepare ethanoic acid from ethanol.

What was the purpose of the condenser?
A to make sure air does not react with ethanol
B to stop ethanoic acid changing back to ethanol
C to stop ethanol being converted into ethane
D to stop ethanol vapour escaping
10. Which compound, on combustion, never forms soot?
A carbon monoxide B ethanol C ethane D methane
11. The molecular structure of a hydrocarbon is shown below.
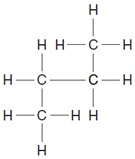
Which structure is an isomer of this hydrocarbon?
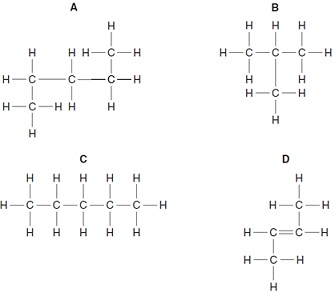
12. Octane is an alkane present in petrol.
What are the products when octane is completely burned in air?
A carbon dioxide and hydrogen
B carbon dioxide and water
C carbon monoxide and water
D carbon monoxide, carbon dioxide and water
13. Which type of reaction occurs when soap is formed from fats?
A hydrolysis B polymerization C fermentation D substitution
14. Which of these equations does not represent an addition reaction?
A CH2Cl2 + Cl2 → CHCl3 + HCl
B C2H4 + Br2 → C2H4Br2
C nC2H4 → -(CH2 - CH2)-n
D C3H6 + H2O → C3H7OH
15. A polymer has the structure shown.
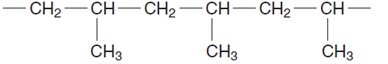
What is the molecular formula of the monomer?
16. What is the linkage between the units in fats and also in Terylene?
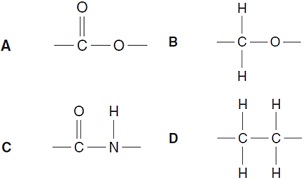
17. The diagram shows, by percentages, the principal large-scale uses of chlorine.
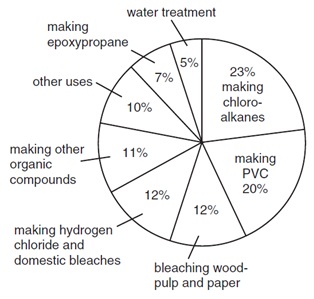
What is the total pecentage of chlorine used in making organic compounds?
A 34% B 54% C 61% D 73%
18. Which product is not manufactured using calcium carbonate?
A cement B chlorine C glass D iron
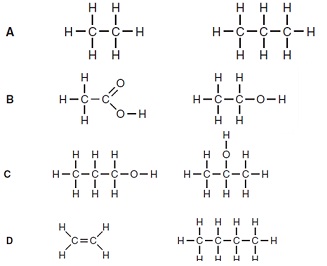
19. Which pair of structures are isomers of each other?
20. The diagrams show the structures of three hydrocarbons.
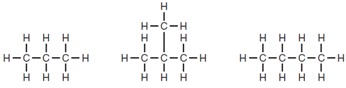
Which statement is correct for all three compounds?
A They are isomers of each other.
B They have the same general formula.
C They have the same physical properties.
D They react with aqueous bromine.