Reference no: EM13698951
1. Using the following data, calculate the Rf values for each of the components. What conclusions can be drawn from this data?
|
Sample
|
Distance of Analyte (mm)
|
Distance of Solvent (mm)
|
|
Unknown
|
53
64
|
92
92
|
|
?9 - THC
|
52
|
90
|
|
Cannabinol
|
61
|
89
|
|
Cannabidiol
|
70
|
89
|
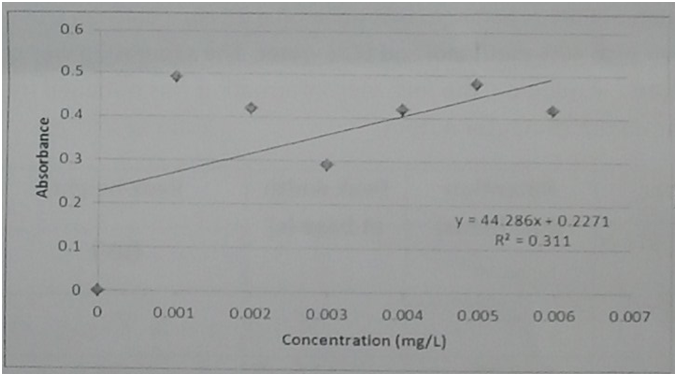
2. Comment on the quality of the following calibration curve and propose how you might improve on it.
3. A compound called "bisphenol A" is used in the manufacture of plastic bottles/containers and in the internal coating of food cans.
You have been asked to establish if BPA added to babies' bottles is leaching into milk.
a) What samples that should be collected (and how)?
b) What sample preparation techniques might you consider using?
c) What type of analysis could be carried out on the samples and using what technique (Where appropriate)?
4. What is sample preparation and why is it necessary?
5. To determine the concentration of Alcohol in an individual's blood sample, a calibration curve was constructed. The concentrations of the standards were in the range 0 - 240 mg/100mL and the equation of the line was found to be y = 0.0545x - 0.06 by using the concentration on the x - axis and the ratio of ethanol to internal standard peak areason the y - axis. The R2value was found to be 0.9998. Determine the concentration of alcohol in a blood sample if the ratio of alcohol to internal standard was found to be 10.0.
6. The separation of FIVE substances was done on a 25cm x 0.46cm C18 column using isocratic elution with 40% methanol and 60% water. The separation was complete in approx. 5 minutes.
In addition, the following information was obtained from the chromatogram via the computer output.
|
Peak number
|
Retention time (mins)
|
Peak width at base (s)
|
Peak height
(µV)
|
Peak area (µV.min)
|
|
0
|
1.20
|
-
|
-
|
-
|
|
1
|
3.40
|
12
|
12884
|
17121
|
|
2
|
3.70
|
7
|
12258
|
14316
|
|
3
|
4.40
|
12
|
10658
|
18409
|
|
4
|
5.30
|
15
|
12530
|
19193
|
|
5
|
6.00
|
6
|
9361
|
16447
|
a) Calculate the capacity factor (K) for each peak using the following equation:
k = tR - t0/t0
b) Why is it better to convert to a capacity factor?
c) Assess the resolution between the following parts of peaks 1 to 2, 2 to 3, 3 to 4 and 4 to 5.
Using the following equation, are each of the pairs of peaksseparated adequately or not?
Rs = tRB - tRA/0.5(WA + WB)
7. The absorbance of a series of calibration samples (concentration range 0 - 500 mg/L) were measured and a calibration curve obtained.
The equation of the straight line was found to be y = 0.0018x - 0.0114 and R2 value found to be 0.9975.
Given that the sample was analyzed in duplicate and the following absorbance values were found, determine the concentration of the sample:
|
Sample Reading
|
Abs @ 254 nm
|
|
1
|
0.414
|
|
2
|
0.416
|
8. The absorbance of a 4.29 x10-5 M solution of a compound is 0.729 at a wavelength of 266 nm in a 1cm cuvette. Calculate the molar absorptivity (ε).(Show your working)
9. Giving the following information, determine the concentration of the samples:(Show your working)
a) A = 0.231 @ 365 nm; ε = 2500 M-1cm-1; l = 1cm
b) A = 0.429 @ 254 nm; ε = 1500 M-1cm-1; l = 1cm
c) A = 0.615 @ 365 nm; ε = 1000 M-1cm-1; l = 1cm
|
Method of dividing toys ensures-final division is efficient
: A parent has a pile of toys to divide between two children. Assume that there is an even number of each toy. What method of dividing the toys ensures that the final division is efficient?
|
|
Describe the business and its likely main competitors
: Assume that you are going to start a small business of your own. Further, imagine that you are able to adequately differentiate your product, or service so that you can establish your business as a monopolistically competitive firm. Describe the busi..
|
|
Budget curve and indifference curves
: Assume consumer equilibrium (budget curve and indifference curves). Draw 3 separate scenarios: (1) the price of good x decreases; (2) the price of good y increases; and (3) the budget (income increases). In each scenario, explain the effect on the gr..
|
|
income elasticity of demand for Art Deco lawn furniture
: Your firm’s research department has estimated the income elasticity of demand for Art Deco lawn furniture to be -0.85. You have just learned that due to an upturn in the economy, consumer incomes are expected to rise by 5 percent next year. How will ..
|
|
What sample preparation techniques might you consider using
: What samples that should be collected and what sample preparation techniques might you consider using - what type of analysis could be carried out on the samples and using what technique.
|
|
What should you do with your stock of inventories
: The income elasticity of demand for your firm’s product is estimated to be 0.75. A recent report in The Wall Street Journal says that national income is expected to decline by 3 percent this year. What should you do with your stock of inventories?
|
|
Which divisions are efficient
: Suppose that one must divide $1,000 among 100 students. Which divisions are efficient?
|
|
A merger between two firms is legal
: Roughly speaking, a merger between two firms is legal:
|
|
What is the social surplus created by eradicating polio
: Suppose that the cost of eradicating polio from a society of 1,000 persons is $5 per person. Also suppose that only two persons in the society will benefit from that policy, and the benefit to each of those persons is $2,000. Then what is the social ..
|