Reference no: EM131214727
Assignment
Instructions:
1. If k1 > k2, is the overall reaction SN1 or SN2?
2. Conversely, if k2 > k1 is the overall reaction SN1 or SN2? Explain why.
The following molecule is chloroethane.
ClCH2CH3
molecule 1
It is unreactive towards hydroxide and most nucleophiles under normal conditions, i.e., the chlorine-carbon bond is not a very good electrophilic center, hence 1 is not a good alkylating agent and does not transfer the CH3CH2 group easily.
On the other hand, there is a class of drugs that contain a chloroethyl group which are good alkylating agents and have been used clinically to treat cancer. An example of this class of drugs is melphalan.
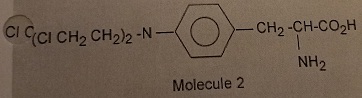
The reason melphalan, is a good alkylating agent, and 1 is not, is due to the formation of the intermediate 3 caused by the close proximity of a basic nitrogen to the carbon-chlorine bond in 2. Reaction of 3 with a nucleophile (Nuc) gives the alkylated nucleophile, 4.
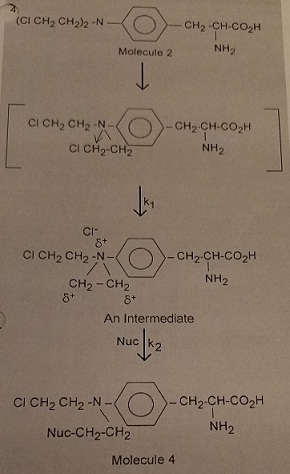
In vivo, in a therapeutic setting, the Nuc is specifically nitrogen or oxygen on a purine in a DNA strand, which once alkylated cleaves from the DNA strand to create errors in reading the DNA code and ultimately death of a cancer cell. The alkylation takes place in two steps defined by the rate constants k1 and k2.
3. If the basic pKa of the nitrogen in the -N-(CH2 - CH2 - Cl)2 in molecule 2 is 1.5 and the basic pKa in the -N-(CH2- CH2 - Cl)2 in Molecule 5 is 6.5, which drug, (molecule 2 or molecule 5) is more likely to go by an SN1 reaction with the nucleophile and why? Note that the rate or ease of reaction k1 for 2 → 3 will depend on how basic is the nitrogen in -N-(CH2 - CH2 -Cl)2
(Cl CH2 CH2.)2 N-CH3
molecule 5
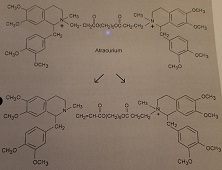
The following molecule, atracurium, is a very complex drug. It is a neuromuscular blocking agent and is related to the natural product tubocurarine. Atracurium exhibits a very short half-life (20-min). The products of the fastest inactivation process are shown below.
4. Research and Explain what chemical mechanism is responsible for the formation of the products.
Hint: The acidic pKa of CH3(C=O)CH3 → CH3(C=O)CH2- is 20.=
|
Find the maximum and minimum voltage on the line
: If the voltage at the load is 100 V, find the maximum and minimum voltage on the line.
|
|
Original array in place in time nk log
: Write down pseudo code for an algorithm that sorts the original array in place in time nk log(k).Your algorithm can use a function sort(A, l, r) that sorts the subarray A[l], . . . , A[r].
|
|
Identify various social trends that affect marketing
: To complete this assignment, students must prepare a real-world marketing news example, event, or success story and communicate it to the class. Students should use popular resources such as The Wall Street Journal, Bloomberg Businessweek, Inc., e..
|
|
Analyse principles and trends in performance
: Performance Management and Control - Identify and critically analyse principles and trends in Performance Measurement and Control and Determine the different budgeting techniques and critically evaluate their use in short term decision making
|
|
What mechanism is responsible for the formation of products
: If k1 > k2, is the overall reaction SN1 or SN2? Research and Explain what chemical mechanism is responsible for the formation of the products.
|
|
Calculate and plot the line impedance as a function
: At the frequency at which the line operates, the reactance of the load is -j50 Ω and the phase constant on the line is 20π rad/m. Calculate and plot the line impedance as a function of distance from load.
|
|
Explain the trade-offs between system scalability
: Explain the trade-offs between system scalability and system transparency you may encounter when designing a large-scale system.
|
|
Plot the line impedance as a function of distance
: At the frequency at which the line operates, the reactance of the load is -j50 Ω and the phase constant on the line is 20π rad/m. Calculate and plot the line impedance as a function of distance from load.
|
|
Why is it an important concept in oop
: 1. Why is it an important concept in OOP? 2. What is the difference between a derived and base class?
|